February 6, 2013
Asahi Kasei Corp.
Asahi Kasei ZOLL Medical Corp.
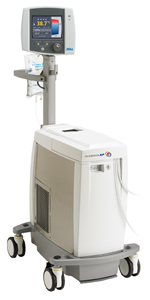
Asahi Kasei ZOLL Medical (AZM), the Asahi Kasei Group company for the sale of critical care devices and systems in Japan, begins selling the Thermogard System™ today, February 6, 2013. A central venous placement temperature management system for the reduction of fever due to neurological injury, this represents a major breakthrough as the first intravascular temperature management (IVTM) system—which controls a patient’s temperature using a central venous catheter—to be available in Japan.
Established in August 2012, AZM is the subsidiary of ZOLL Medical Corporation which handles matters related to the regulatory approval, sale, and marketing of ZOLL products in Japan. The Thermogard System™, for which Japanese regulatory approval was obtained in June 2012, is ZOLL’s first product launched in Japan since joining the Asahi Kasei Group in April 2012.
AZM will continue to advance preparations for regulatory approval of other ZOLL products such as the LifeVest™ wearable defibrillator, while working together with other Asahi Kasei Group companies to expand operations in the field of critical care.
About the Thermogard System™
Purpose of use:
Neurogenic fever (fever caused by abnormalities in the temperature regulating center of the hypothalamus) is common in neurological injury such as ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and traumatic brain injury, and is associated with increased mortality and poor outcome. The Thermogard System™ is used for therapeutic normothermia for neurologically injured patients, with a unique multi-balloon catheter placed into a central vein (superior or inferior vena cava) which regulates body temperature by heat exchange with blood.
Characteristics:
Temperature-controlled saline is circulated within a closed-loop multi-balloon catheter placed into a central vein, and the patient is cooled as blood passes over each balloon. The system regulates the temperature of the circulating intra-balloon saline automatically based on feedback of the patient’s core temperature, enabling precise temperature management with minimal nursing workload.
Other potential indications:
AZM is advancing preparations for clinical studies for therapeutic hypothermia following cardiopulmonary resuscitation, a treatment having Class I recommendation* from both the American Heart Association (AHA) and the Japan Resuscitation Council (JRC).
Adobe Readeris required to view these PDF files.