December 9, 2013
Asahi Kasei Corp.
Asahi Kasei ZOLL Medical Corp.
Asahi Kasei ZOLL Medical (AZM), the Asahi Kasei Group company for critical care devices in Japan, received approval on November 22, 2013, from Japan’s Ministry of Health, Labor and Welfare for the manufacturing and marketing of the X Series® defibrillator as Designated Marketing Authorization Holder.
The X Series®, a portable defibrillator that includes monitoring functions, is suited for use in a wide range of circumstances at the scene of an emergency, during patient transport, and within medical facilities. It features light weight and a compact form without compromising high functionality and large display size. In addition to defibrillation, it provides external pacing as well as monitoring of electrocardiograph (ECG), invasive and non-invasive blood pressure, pulse oximetry (including SpO2, CO, etc.), end-tidal carbon dioxide concentration (EtCO2), body temperature, and respiratory rate.
Designed for use under severe on-site conditions during emergencies, the X Series® is highly rugged and durable, with a dustproof, waterproof enclosure meeting the IP55 standard. It also features advanced capability to communicate data from its monitoring functions to the hospital from the emergency site and during patient transport.
In addition, the X Series® incorporates the Real CPR Help® function, a proprietary technology of AZM’s parent company ZOLL Medical Corporation. This provides real-time feedback on rate and depth of compressions, helping rescuers perform higher-quality cardiopulmonary resuscitation (CPR). Its See-Thru CPR® function furthermore filters out compression artifact on the ECG monitor so that rescuers can see the underlying heart rhythm during CPR, thereby reducing the duration of pauses in compressions.
AZM is now advancing preparations for sale of the X Series® in Japan, with launch planned for spring 2014.
Outline of the approval
| Approval number: | 22500BZI00025000 | |
|---|---|---|
| Foreign Manufactured Medical Device’s Manufacturing and Marketing Approval Holder: | ZOLL Medical Corporation (US) | |
| Designated Marketing Authorization Holder: | Asahi Kasei ZOLL Medical Corp. |
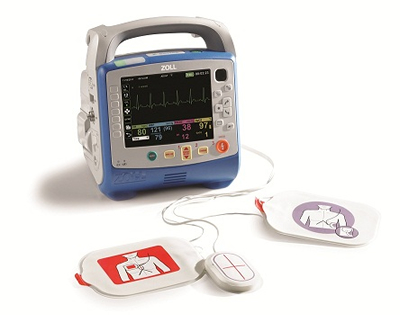
X Series® defibrillator
About Asahi Kasei ZOLL Medical (AZM)
A subsidiary of Asahi Kasei Group company ZOLL Medical Corporation, AZM was established in August 2012 to sell ZOLL critical care products in Japan. AZM will continue to seek regulatory approval for other ZOLL products for their sale in Japan, while working to achieve synergy with the other health care operations of the Asahi Kasei Group.
Adobe Readeris required to view these PDF files.