December 16, 2014
Asahi Kasei Fibers Corp.
Asahi Kasei Fibers has developed NanoAct™ cellulose nanobeads as labels for lateral flow immunoassay and other assay applications. The properties of cellulose such as excellent colorability and hydrophilicity make NanoAct™ ideal for use as labels in immunochromatography*. Compared to conventional labels, NanoAct・features higher sensitivity, faster detection time, and availability in multiple colors to allow easy multiplexing.
Asahi Kasei Fibers has begun commercial production and supply of NanoAct™ cellulose nanobeads, and is now accelerating the development of lateral flow immunoassays in Japan and worldwide.
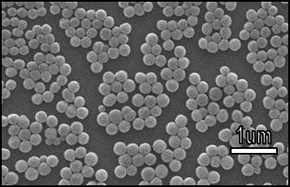
Magnified image of NanoAct™

NanoAct™ product lineup
Background
Asahi Kasei Fibers developed NanoAct™ utilizing the company’s nanotechnology and deep dyeing technology based on cellulose processing know-how accumulated through 80 years of experience in Bemberg™ cupro fiber.
While conventional labels experience drawbacks such as difficulty of detecting antigens at low concentration, slow detection time, and lack of colorability, NanoAct™ provides superior performance to successfully overcome each of these drawbacks. In recognition of these advantages, NanoAct™ has been adopted as the label for an influenza A and B assay that went on sale in Japan on December 15, 2014. The new assay using NanoAct™ is manufactured by Adtec Co., Ltd., and sold by LSI Medience Corp. Other diagnostic assays using NanoAct™ are being developed in Japan and around the world, with market launch expected from 2015 onward.
Features of NanoAct™
NanoAct™ has notable advantages over labels such as colloidal gold and colored latex, which are conventionally used in lateral flow immunoassay.
1.Better visibility
Although colloidal gold particles can produce deep color, they have small surface area. Whereas latex has large surface area, it can’t produce deep color. In contrast, NanoAct™ combines both intense color and large particle size.
2.Higher sensitivity
Since NanoAct™ has superior visibility, it enables better detection of low antigen concentration than conventional labels. In performance comparisons based on experimental models using human chorionic gonadotropin (hCG) and troponin I* assays, the sensitivity of NanoAct™ was 8 to 10 times higher than that of colloidal gold. Higher sensitivity means fewer false negatives due to antigen concentration being below the detectable limit, and will contribute to early diagnosis. It furthermore enables reduced physical discomfort for patients through the use of saliva or other low-concentration samples rather than throat swabs or blood samples.
3.Faster detection time
With its outstanding visibility and sensitivity, NanoAct™ enables shorter detection time. Patients can have shorter waiting times, and caregivers can efficiently see more patients.
4.Multiple colors
NanoAct™ is available in 6 colors: 2 shades of red, 2 shades of blue, 1 shade of green, and black. The color variation enables multi-analyte detection.
NanoAct™ product outline
| Release date: | Mid-December 2014 | |
|---|---|---|
| Particle diameter: | 330 nm (average) | |
| Colors: | 2 red, 2 blue, green, black | |
| Optical density: | Maximum 270 (varies with color) | |
| Solvent: | Water | |
| Concentration: | 1% by weight | |
| Sales target: | ¥1 billion within several years |
Adobe Readeris required to view these PDF files.