L–α–GLYCEROPHOSPHATE OXIDASE [GPOM]
from Streptococcus sp.
(sn–Glycerol–3–phosphate: oxygen 2–oxidoreductase, EC 1. 1. 3. 21)
L–α–Glycerophosphate + O2 → Dihydroxyacetone phosphate + H2O2
Preparation and Specification
- Appearance
- : Yellowish amorphous powder, lyophilized
- Specific activity
- : More than 15.0 U/mg solid
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 169 kDa (TSK gel G 3000 SW XL gel filtration)
65 kDa (SDS–PAGE)
- Isoelectric point
- : pH 4.4
- Michaelis constants
- : L–α–Glycerophosphate 0.64 mM (pH 7.5)
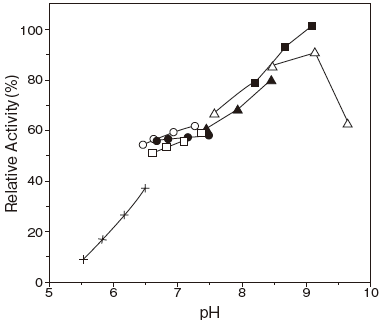
- Optimum pH
- : 8.5–9.0Figure 1
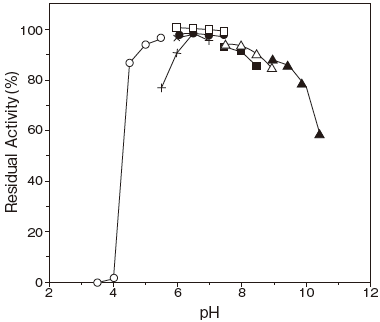
- pH stability
- : 6.0–8.0 (37℃, 30 min) Figure 2
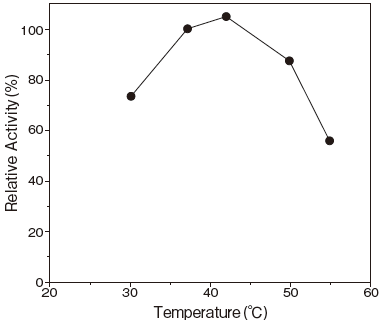
- Optimum temperature
- : 37–42℃ (pH 6.5) Figure3
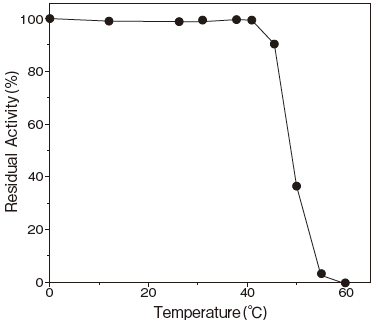
- Thermal stability
- : Stable at 40℃ and below (pH 6.5) Figure4
- Effect of metal ions
- : See Table 2
- Effect of detergents
- : See Table 3
- tabilizers
- : FAD
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of triglyceride.
| LP | ||
| TG + 3 H2O | → | Glycerol + 3 Fatty acid |
| GKZ | ||
| Glycerol + ATP | → | G-3-P + ADP |
| GPOM | ||
| G-3-P + O2 | → | DHAP + H2O2 |
| POD | ||
| 2 H2O2 + 4-AA + Phenol | → | Quinoneimine dye + 4 H2O |
TG: Triglyceride, DHAP: Dihydroxyacetone phosphate
Table 1. Substrate specificity
| Substrate (300mM) | Relative
activity (%) |
|---|---|
| L–α–Glycerophosphate | 100 |
| Glucose–1–phosphate | 0 |
| Glucose–6–phosphate | 0 |
| Glycerol | 0 |
| Glucose | 0 |
Table 3. Effect of detergents on GPOM activity
| Detergent (0.1%) | Relative
activity (%) |
|---|---|
| None | 100 |
| EMULGEN 810 | 98 |
| EMULGEN 911 | 98 |
| RHEODOL TWL–106 | 99 |
| RHEODOL 460 | 99 |
| ADEKANOL NP–720 | 99 |
| Triton X–100 | 98 |
| Triton X–305 | 99 |
| Tween 80 | 98 |
Table 2. Effect of metal ion on GPOM activity
| Metal ion (2mM) | Relative
activity (%) |
|---|---|
| None | 100 |
| MgCl2 | 101 |
| MgSO4 | 102 |
| ZnCl2 | 102 |
| ZnSO4 | 102 |
| NaCl | 103 |
| NH4Cl | 103 |
| BaCl2 | 103 |
| Ba (CH3COO) 2 | 101 |
| NiCl2 | 103 |
| CoCl2 | 103 |
| MnCl2 | 114 |
| LiCl | 103 |
| KCl | 102 |
| CaCl2 | 103 |
Assay
Principle
-
The assay is based on the increase in absorbance at 600 nm as the formation of quinoneimine dye in the following reactions:
| GPOM | ||
| L–α–Glycerophosphate+O2 | → | Dihydroxyacetone phosphate+H2O2 |
| POD | ||
| 2 H2O2+4–AA+DAOS | → | Quinoneimine dye+4H2O |
DAOS : [3,5–dimethoxy–N–ethyl–N– (2–hydroxy–3– sulphopropyl) aniline]
-
Unit definition
-
One unit is defined as the amount of enzyme which generates 1 μmole of H2O2 per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture
Dissolve 6.05 g of PIPES and 9.45 g (purity calculation) of Disodium Glycerophosphate with 70 ml of distilled water and adjust pH to 6.5 with 4 N NaOH at 25℃. Add all reagents listed below and confirm pH is 6.5 at 25℃.
Add distilled water to make a total of 100 ml.100 U/ml POD 1) solution 5.0 ml 15 mM 4–AA solution 10.0 ml
-
-
100mM DAOS solution 1.0 ml 5% (W/V) Triton X–100 solution 1.0 ml 1) : 100 U/ml POD solution Dissolve 1,000 U (PPU) of POD with 10 ml of distilled water. - Reaction stopper
0.5% (W/V) SDS solution
SDS: Sodium dodecyl sulfate - Enzyme dilution buffer
10 mM PIPES–NaOH buffer pH 6.5
containing 0.1% (W/V) Triton X–100 - Reagents
PIPES [Piperazine–1,4,–bis (2–ethanesulfonic acid) ]:Dojindo Laboratories # 345–02225DAOS (sodium salt) : Dojindo Laboratories #OC06
4–AA: NACALAI TESQUE, INC.Special grade #01907–52Triton X–100: The Dow Chemical Company
Disodium Glycerophosphate 5.5 Hydrate :FUJIFILM Wako Pure Chemical CorporationSDS (Sodium Dodecyl Sulfate) :
#192–02055NACALAI TESQUE, INC. Extra pure #31606–75POD: Sigma Chemical Co. Type Ⅱ #P–8250
Enzyme solution
- Accurately weigh about 20 mg of the sample and add enzyme dilution buffer to make a total of 20ml. Dilute it with enzyme dilution buffer to adjust the concentration as required.
Procedure
- Pipette accurately 1.0 ml of reaction mixture into a
small test tube and preincubate at 37℃. - After 5 min, add exactly 20 μl of enzyme solution and mix to start the reaction at 37℃.
※ In the case of a test blank, add 20 μl of enzyme dilution buffer in place of enzyme solution. - At 5 min after starting the reaction, add 2.0 ml of the
- reaction stopper to stop the reaction.
- Measure the absorbance at 600 nm.
0.1 Abs ≦ △A (As−Ab) ≦ 0.2 AbsAbsorbance sample : As blank : Ab
Calculation
Activity (U/mg of powder) = {(△ A/5)/(16.8×1/2)} × 3.02/0.02 × 1/x| 16.8 : | millimolar extinction coefficient of quinoneimine dye at 600 nm
( cm2 /μmole)
|
| 1/2 : | a multiplier derived from the fact that 2 mole of H2O2 produces l mole of quinoneimine dye |
| 5 : | reaction time (min) |
| 3.02 : | final volume (ml) |
| 0.02 : | volume of enzyme solution (ml) |
| X : | concentration of the sample in enzyme solution
( mg/ml)
|
Storage
Storage at −20℃ in the presence of a desiccant is recommended. Enzyme activity will be retained for at least one year under this condition.
References
- Jacobs, N. J. and Van Demark, P. J. (1960) Arch.
Biochem. Biophys., 88, 250–255. - Koditschek, L. K. and Umbreit, W. W. (1969) J. Bacteriol., 98, 1063–1068.
- Gancedo, C., Gancedo, J. M. and Sols, A. (1968) J. Biochem. ( Tokyo) , 5, 165–172.
- Kistler, W. S., Hirsch, C. A., Cozzarelli, N. R. and Lin, E. C. C. (1969) J. Bacteriol., 100, 1133–1135.
- Esders, T. W. and Michrina, C. A. (1979) J. Biol. Chem., 254, 2710–2715.
GPOM 活性測定法 (Japanese)
試薬液
- 反応試薬混合液
PIPES 6.05g とグリセロりん酸2Na 9.45g (純度換算) を精製水70ml に溶解した後、4N NaOH でpH6.5 (25℃) に調整し、その液に下記試薬を加えて混和し、pH6.5 (25℃) であることを確認した後、精製水で全容100ml とする。100U/ml POD 溶液1) 5.0 ml 15mM 4–AA 溶液 10.0 ml 100mM DAOS 溶液 1.0 ml 5% (W/V) トリトンX–100 溶液 1.0 ml 1) : 100U/ml POD 溶液
POD 1,000 単位 (PPU) を精製水10ml で溶解する。
- 反応停止液
0.5% (W/V) SDS 溶液 - 酵素溶解希釈用液
0 . 1 % ( W / V ) トリトン X – 1 0 0 を含む1 0 m M
PIPES–NaOH 緩衝液 pH6.5 - 試薬
PIPES[ピペラジン–1,4–ビス (2–エタンスルホン酸) ]:同仁化学製 #345–02225
DAOS[3,5–ジメトキシ–N–エチル–N– (2–ヒドロキ
シ–3–スルフォプロピル) アニリン]:同仁化学製 #OC064–AA:ナカライテスク製 特級 #01907–52
トリトンX–100:Dow Chemical 製
グリセロりん酸二ナトリウム5.5 水和物:富士フイルム和光純薬製 #192–02055SDS (ドデシル硫酸ナトリウム) :ナカライテスク製 一級 #31606–75POD:シグマ製 Type Ⅱ #P–8250
酵素試料液
- 検品約20mg を精密に量り、酵素溶解希釈用液で溶解して全容20ml とする。
その液を酵素溶解希釈用液で適宜希釈する。
測定操作法
- 小試験管に反応試薬混合液1.0ml を正確に分注し、37℃で予備加温する。
- 5 分経過後、酵素試料液20 μl を正確に加えて混和し、37℃で反応を開始する。
※ 盲検は酵素試料液の代わりに酵素溶解希釈用液
20 μl を加える。 - 5 分経過後、反応停止液2.0ml を加えて混和し、反応を停止する。
- 600nm における吸光度を測定する。
求められた吸光度を試料液はAs、盲検液はAb とする。
0.1 Abs ≦ ΔA = (As−Ab) ≦ 0.2 Abs
計算
-
以下の計算式に従い、活性 (U/mg) を計算する。
活性 (U/mg) = {(△A/5)/(16.8×1/2)} × 3.02/0.02 × 1/x
-
16.8 : キノンイミン色素の600nm におけるミリモル分子吸光係数 (cm2 / μmole)1/2 : H2O2 2 モルからキノン色素1 モルが生成することによる係数 5 : 反応時間 (min) 3.02 : 反応総液量 (ml) 0.02 : 反応に供した酵素試料液量 (ml) X : 酵素試料液中の検品濃度 (mg/ml)
Copyright © Asahi Kasei Pharma Corporation. All rights reserved.