MONOGLYCERIDE LIPASE [MGLPⅡ]
from Bacillus sp.
(Glycerol–ester hydrolase, EC 3.1.1.23)
Monoacylglycerol + H2O → Glycerol + Fatty Acid
Preparation and Specification
- Appearance
- : White amorphous powder, lyophilized
- Specific activity
- : More than 20 U/mg solid
- Contaminants
- :
- Catalase
- Less than 0.5% (U/U)
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 20 kDa (gel filtration)
- Isoelectric point
- : pH 4.8±0.2
- Michaelis constants
- : Monolaurine 1.8 × 10-4M
- Optimum pH
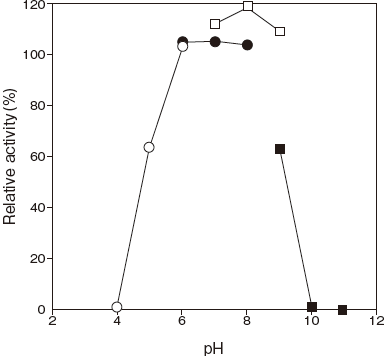
- : 6.0–8.0Figure 1
- pH stability
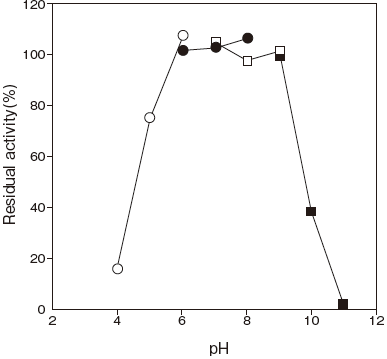
- : 6.0–8.0 (65℃, 10 min) Figure 2
- Optimum temperature
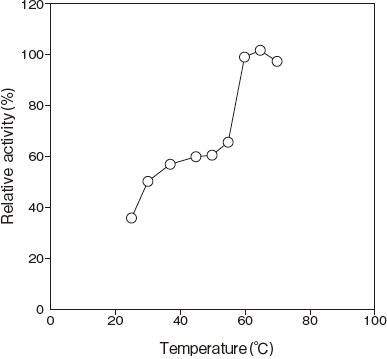
- : 65℃ (PIPES buffer) Figure3
- Thermal stability
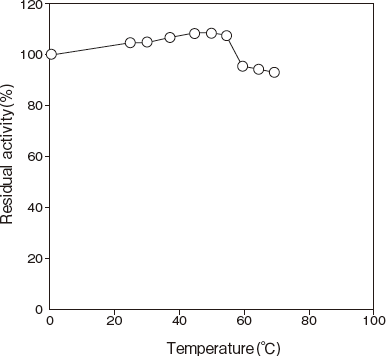
- : Stable at 65℃ and below (pH 8.0, 10 min) Figure4
- Effect of metal ions
- : See Table 2
- Effect of detergents
- : See Table 3
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of triglyceride.
| LP + MGLP Ⅱ | ||
| TG + 3 H2O | → | Glycerol + 3 FFA |
TG: Triglyceride
FFA: Free fatty acid
Table 1. Substrate specificity
| Substrate | Relative activity (%) |
|---|---|
| 1–Monocaprylin | 81.8 |
| 1–Monolaurin | 100 |
| 1–Monomyristin | 96.3 |
| 1–Monopalmitin | 66.3 |
| 1–Monostearin | 31.0 |
| 1–Monoolein | 62.3 |
| 1–Monolinolein | 110 |
| Triolein | 0 |
Table 2. Effect of metal ions on MGLP Ⅱ activity
| Metal ion (10mM) |
Relative activity (%) |
|---|---|
| None | 100 |
| NaCl | 83.0 |
| KCl | 79.0 |
| LiCl | 78.0 |
| MgCl2 | 77.0 |
| MnCl2 | 77.0 |
| CaCl2 | 78.0 |
Table 3. Effect of detergents on MGLP Ⅱ activity
| Detergent (0.1%) |
Relative activity (%) |
|---|---|
| None | 100 |
| Triton X–100 | 67.0 |
| Triton X–114 | 67.0 |
| Adekanol 795 | 64.0 |
| Emulgen B–66 | 67.0 |
| Emulgen 911 | 65.0 |
| Emulgen 810 | 66.0 |
| Emulgen 460 | 61.0 |
| Rheodol TWL–106 | 67.0 |
Assay
Principle
The assay is based on the increase in absorbance at 550 nm as the formation of quinoneimine dye proceeds in the following reactions:
| MGLP Ⅱ | ||
| Monolaurin+H2O | → | Glycerol+Lauric acid |
| GK | ||
| Glycerol+ATP | → | Glycerol–3–P+ADP |
| GPOSP | ||
| Glycerol–3–P+O2 | → | Dihydroxyacetone phosphate+H2O2 |
| POD | ||
| 2 H2O2+4–AA+TOOS | → | Quinoneimine dye+4 H2O |
ATP : Adenosine triphosphate
GK : Glycerol kinase
GPOSP: Glycerophosphate oxidase
TOOS: Ethyl–N– (2–hydroxy–3–sulfopropyl) –m–toluidine sodium salt,
Unit definition
-
One unit is defined as the amount of enzyme which liberates 1 μmole of monoglyceride per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture
0.2 M PIPES–NaOH buffer pH 7.3 0.10 ml 15mM 4–AA solution 0.05 ml 0.3% (W/V) TOOS solution 0.05 ml 100 U/ml POD solution 1) 0.025 ml 100 mM MgCl2 solution 0.005 ml 50 mM ATP solution pH7.0 0.01 ml 25 U/ml GK solution 2) 0.01 ml
-
150 U/ml GPOSP solution 3) 0.10 ml Distilled water 0.05 ml 1) : 100 U/ml POD solution
Dissolve 1,000 U (PPU) of POD with 10 ml of distilled water.2) : 25 U/ml GK solution
Dissolve 250 U of GK with 10 ml of distilled water.3) : 150 U/ml GPOSP solution
1,500 U of GPOSP with 10 ml of distilled water. - Substrate solution
(1) Substrate preparation buffer
5 mM MES–NaOH buffer pH 5.5 containing 0.5% (W/V) Triton X–100 MES [2– (N–monophoryno) ethanesulfonic acid monohydrate](2) Substrate solution (for stock)
0.5M Monolaurine–ethanol solution(3) Substrate solution (milky colored)
Mix 0.2 ml of substrate solution (for stock) and 9.8 ml of substrate preparation buffer - Reaction stopper
0.5% (W/V) SDS solution
SDS: Sodium dodecyl sulfate - Enzyme dilution buffer
10 mM PIPES–NaOH buffer pH 7.3 containing 0.1% (W/V) BSA - Reagents
PIPES [Piperazine–1,4–bis (2–ethanesulfonic acid) ]:Dojindo Laboratories #345–02225TOOS: Dojindo Laboratories #OC13
MES: Dojindo Laboratories #349–01623
BSA: Millipore Fraction V pH5.2 #81–053
Monolaurin: Tokyo Kasei Kogyo Co., Ltd #G0081
GK: Asahi Kasei Pharma Corporation #T–09
GPOSP: Asahi Kasei Pharma Corporation #T–60
4–AA: NACALAI TESQUE, INC. Special grade#01907–52Triton X–100: The Dow Chemical Company
SDS: NACALAI TESQUE, INC. #316–06
POD: Sigma Chemical Co. Type Ⅱ #P–8250
Enzyme solution
-
Accurately weigh about 20 mg of the sample and add enzyme dilution buffer to make a total of 20 ml. Dilute it with enzyme dilution buffer to adjust the concentration as required.
Procedure
- Pipette accurately 0.40 ml of reaction mixture and 50 μl of substrate solution into a small test tube and preincubate at 37℃.
- After 3 min, add exactly 20 μl of enzyme solution and mix to start the reaction at 37℃.
※ In the case of a test blank, add 20 μl of enzyme dilution buffer in place of enzyme solution. - At 10 minutes after starting the reaction, add 2.0 ml of the reaction stopper to stop the reaction.
- Measure the absorbance at 550 nm.
-
△A = (As−Ab) ≦ 0.700AbsAbsorbance sample : As blank : Ab
Calculation
-
Activity (U/mg of powder) = {(△ A/10)/(15.6)} × 2.47/0.02 × 1/x
15.6 : millimolar extinction coefficient of quinoneimine dye
at 550 nm (cm2/ μmole)10 : reaction time (min) 2.47 : final volume (ml) 0.02 : volume of enzyme solution (ml) X : concentration of the sample in enzyme solution ( mg/ml)
Storage
-
Storage at −20℃ in the presence of a desiccant is recommended.
References
-
Imamura, S., and Kitaura, S (. 2000) J. Biochem. ( Tokyo) , 127, 419–425.
MGLP Ⅱ活性測定法 (Japanese)
試薬液
- 反応試薬混合液
0.2M PIPES–NaOH 緩衝液 pH7.3 0.10 ml 15mM 4–AA 溶液 0.05 ml 0.3% (W/V) TOOS 溶液 0.05 ml 100U/ml POD 溶液 1) 0.025 ml 100mM 塩化マグネシウム溶液 0.005 ml 50mM ATP 溶液 pH7.0 0.01 ml 25U/ml GK 溶液 2) 0.01 ml 150U/ml GPOSP 溶液 3) 0.10 ml 精製水 0.05 ml
-
1) : 100U/ml POD 溶液
POD 1,000 単位 (PPU) を精製水10ml で解する。2) :
25U/ml GK 溶液
GK 250 単位 (U) を精製水10ml で溶解する。3) : 150U/ml GPOSP 溶液
GPOSP 1,500 単位 (U) を精製水10ml で溶解する。 - 基質溶液
① 基質調製用液
0.5% (W/V) トリトンX–100 を含む5mM
MES–NaOH 緩衝液 pH5.5②
保存基質溶液
0.5M モノラウリンーエタノール溶液
-
③ 基質溶液
保存基質溶液0.2ml と基質調製用液9.8ml を混合 (白濁する) して基質溶液とする。 - 反応停止液
0.5% (W/V) SDS 溶液 - 酵素溶解希釈用液
0.1% (W/V) BSA を含む10mM PIPES–NaOH 緩衝液 pH7.3 - 試薬
PIPES[ピペラジン–1,4–ビス (2–エタンスルホン酸) ]:同仁化学製 #345–02225
TOOS[エチル–N– (2–ヒドロキシ–3–スルフォプロピル) –m–トルイジンナトリウム塩]:同仁化学製#OC13MES[2– (N–モルフォリノ) エタンスルフォン酸モノヒドレート]:同仁化学製 #349–01623
ATP (アデノシン三リン酸・2Na・2H2O) :協和発酵製BSA : Millipore 製 Fraction V pH5.2 #81–053
モノラウリン (Monolaurin) :東京化成工業製 #G0081GK (グリセロールキナーゼ) :旭化成ファーマ製#T–09GPOSP (グリセロリン酸オキシダーゼ) :旭化成ファーマ製 #T–604–AA:ナカライテスク製 特級 #01907–52
トリトンX–100:Dow Chemical 製
SDS (ドデシル硫酸ナトリウム) :ナカライテスク製 #316–06POD:シグマ製 Type Ⅱ #P–8250
酵素試料液
- 検品約20mg を精密に量り、酵素溶解希釈用液で溶解して全容20ml とする。
その液を酵素溶解希釈用液で適宜希釈する。
測定操作法
- 小試験管に反応試薬混合液0.40ml と基質溶液50 μlを正確に分注し、37℃で予備加温する。
- 3 分経過後、酵素試料液20 μl を正確に加えて混和し、37℃で反応を開始する。
※ 盲検は酵素試料液の代りに酵素溶解希釈用液20 μlを加える。 - 10 分経過後、反応停止液2.0ml を加えて混和し、反応を停止する。
- 550nm における吸光度を測定する。
求められた吸光度変化を試料液はAs、盲検液はAbとする。
ΔA = (As−Ab) ≦ 0.700Abs
計算
活性 (U/mg) = {(ΔA/10)/(15.6)} × 2.47/0.02 × 1/x| 15.6 : | キノン色素の550nm におけるミリモル分子吸光係数 (cm2 /μmole) |
| 10 : | 反応時間 (min) |
| 2.47 : | 反応総液量 (ml) |
| 0.02 : | 反応に供した酵素試料液量 (ml) |
| X : | 酵素試料液中の検品濃度 (mg/ml) |