PHOSPHOGLUCOSE ISOMERASE [PGIⅡ]
from Geobacillus stearothermophilus
(D–Glucose–6–phosphate ketol–isomerase, EC 5.3.1.9)
D–Glucose–6–phosphate → D–Fructose–6–phosphate
Preparation and Specification
- Appearance
- : White amorphous powder, lyophilized
- Specific activity
- : More than 250 U/mg solid
Properties
- Molecular weight
- : 200 kDa (gel filtration)
50 kDa (SDS–PAGE)
- Isoelectric point
- : pH 4.3
- Michaelis constants
- : D–Fructose–6–phosphate 0.21 mM (at 37 ℃)
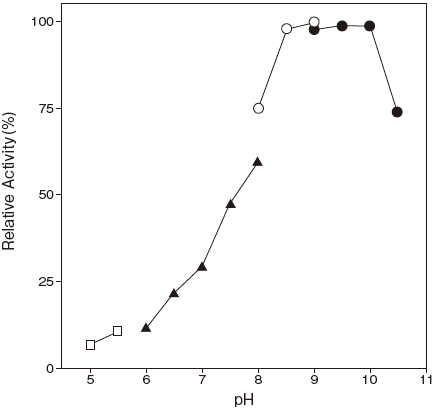
- Optimum pH
- : 9.5Figure 1
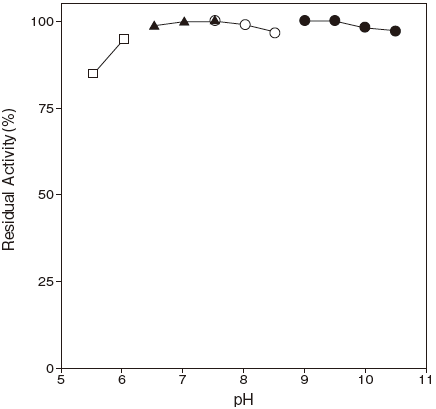
- pH stability
- : 6.5–10.5 (37℃, 1 hr.) Figure 2
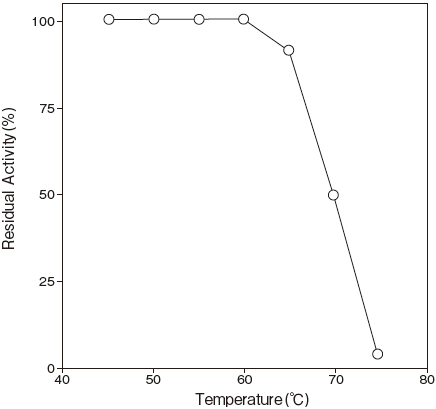
- Thermal stability
- : Stable at 60℃ and belowFigure3
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of F–6–P.
| PGI Ⅱ | ||
| D-Fructose-6-phosphate | → | D-Glucose-6-phosphate |
| G6PDH | ||
| D-Glucose-6-phosphate + NADP+ | → | |
| D-Glucono-δ-lactone-6-phosphate + NADPH + H+ | ||
Assay
Principle
-
The assay is based on the increase in absorbance at 340 nm as the formation of NADPH proceeds in the following reactions:
| PGI Ⅱ | ||
| Fructose–6–P | → | D–Glucose–6–P |
| G6PDH | ||
| D–Glucose–6–P+NADP+ | → | D–Glucono–δ–6–P+NADPH+H+ |
Unit definition
-
One unit is defined as the amount of enzyme which converts 1 μmole of fructose–6–phosphate to D–Glucose–6–phosphate per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture (10test)
0.1M Tris–HCl Buffer pH9.0 28.44 ml 0.1M Fructose–6–P solution 0.90 ml 22.5mM NADP solution 0.60 ml 700U/ml G6PDH suspension 0.06 ml ※ Use G6PDH 10mg/2ml (700U/ml) : market product - Enzyme dilution buffer
50mM Tris–HCl buffer pH8.5 - Reagents
Tris (hydroxymethyl) aminomethane:Sigma Chemical Co. #T–1503Fructose–6–phosphate・2Na:FUJIFILM Wako Pure Chemical CorporationNADP (Nicotinamide adenine dinucleotide phosphate
#066–05341
oxidized form) :FUJIFILM Wako Pure Chemical Corporation #308–50463G6PDH (Glucose–6–phosphate dehydrogenase) :Ammonium sulfate suspension Roche Diagnostics GmbH
#10 127 671 001
Enzyme solution
-
Accurately weigh about 10 mg of the sample and add enzyme dilution buffer to make a total of 10 ml. Dilute it with enzyme dilution buffer to adjust the concentration to 5.0–10.0 U/ml.
Procedure
- Pipette accurately 3.0 ml of reaction mixture into a small test tube and preincubate at 30℃.
- After 5 min add accurately 10 μl of enzyme solution and mix to start the reaction at 30℃.
※ In the case of a test blank, add 10 μl of enzyme dilution buffer in place of enzyme solution. - After starting the reaction, measure the rate of increase per minutes in absorbance at 340 nm. The rate must be measured within the linear portion of the absorbance curve. (Ex. Linear range from 2 min to 5 min)
-
△A/min= (As/min-Ab/min) ≦0.05-0.10Abs/minAbsorbance sample : As/min blank : Ab/min
Calculation
-
Activity (U/mg of powder) = {(△ A/min)/6.22} × 3.01/0.01 × 1/x
6.22 : millimolar extinction coefficient of NADH at 340nm (cm2/ μmol) 3.01 : final volume (ml) 0.01 : volume of enzyme solution (ml) X : concentration of the sample in enzyme solution ( mg/ml)
Storage
-
Storage at −20℃ in the presence of a desiccant is recommended.
PGI Ⅱ活性測定法 (Japanese)
試薬液
- 反応試薬混合液 (10 テスト用)
0.1M トリス–HCl 緩衝液 pH9.0 28.44 ml 0.1M フルクトース–6–P 溶液 0.90 ml 22.5mM NADP 溶液 0.60 ml 700U/ml G6PDH 懸濁液 0.06 ml ※ ロシュ製のG6PDH 10mg/2ml (700U/ml) 3.2M硫安懸濁液をそのまま使用する。 - 酵素溶解用液
50mM トリス–HCl 緩衝液 pH8.5 - 試薬
トリス (ヒドロキシメチル) アミノメタン:シグマ製 #T–1503フルクトース–6– リン酸・2Na:富士フイルム和光純薬製 #066–05341NADP (ニコチンアミドアデニンジヌクレオチド・リン酸酸化型) :富士フイルム和光純薬製 #308–50463G6PDH (グルコース–6–リン酸脱水素酵素) :ロシュ製 #10 127 671 001
酵素試料液
- 検品約10mg を精密に計り、酵素溶解用液にて溶解し全容10ml とする。
その液を更に酵素溶解用液にて5~10U/ml の濃度に適宜希釈する
測定操作法
- 小試験管に反応試薬混合液3.00ml を正確に分注し、30℃で予備加温する。
- 5 分経過後、酵素試料液10 μl を正確に加え混和し、30℃で反応を開始する。
※ 盲検は酵素試料液の代わりに酵素溶解用液10 μlを加える。
- 反応開始後、340nm における吸光度を測定して直線的に反応している1 分間当たりの吸光度変化を求める。 (直線範囲例:2 分目から5 分目)
求めた吸光度変化を
酵素試料液についてはAs/min
盲検液については Ab/min とする。
ΔA/min = (As/min-Ab/min) = 0.05 ~ 0.10/min
計算
以下の計算式に従い、PGI Ⅱ活性 (U/mg) を計算する。活性 (U/mg) = {(ΔA/min)/(6.22)} × 3.01/0.01 × 1/x
| 6.22 : | NADPH の340nm におけるミリモル分子吸光係数 (cm2 /μmol) |
| 3.01 : | 反応総液量 (ml) |
| 0.01 : | 反応に供した酵素試料液量 (ml) |
| X : | 酵素試料液中の検品濃度 (mg/ml) |
Copyright © Asahi Kasei Pharma Corporation. All rights reserved.