ACYL–CoA SYNTHETASE [ACS]
from Pseudomonas fragi
(Acid: CoA ligase (AMP forming) , EC 6.2.1.3)
RCOOH + ATP + CoASH → RCO–SCoA + PPi + AMP
Preparation and Specification
- Appearance
- : White amorphous powder, lyophilized
- Specific activity
- : More than 2 U/mg solid
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 60 kDa ( Sephadex G–150) , 62 kDa ( SDS–PAGE)
- Isoelectric point
- : pH 5.2
- Michaelis constants
- : Palmitic acid 1.1 × 10-5M
ATP 1.7 × 10-4M
CoA 3.2 × 10-4M
- Optimum pH
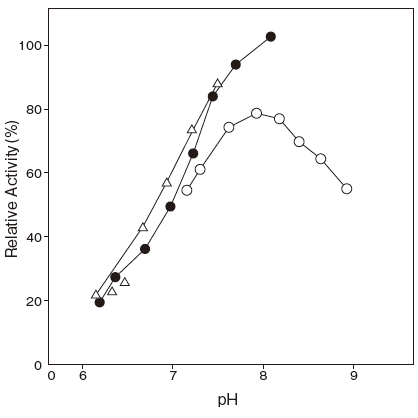
- : Palmitic acid 7.7Figure 1
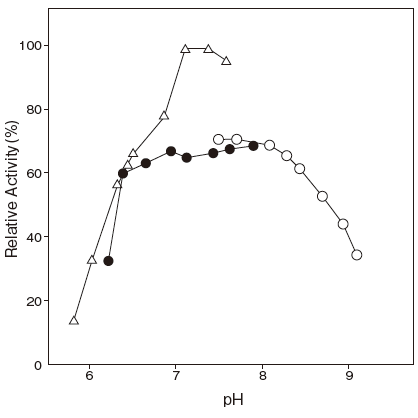
- : Serum fatty acids 7.7Figure 2
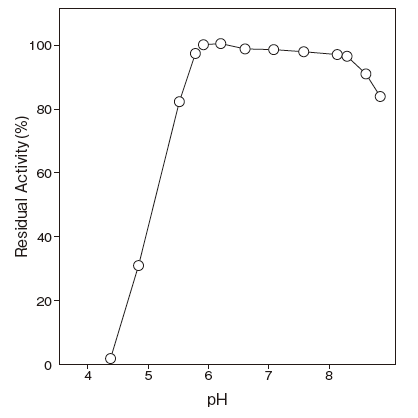
- pH stability
- : 6.0–8.0 (37℃, 2 hr) Figure 3
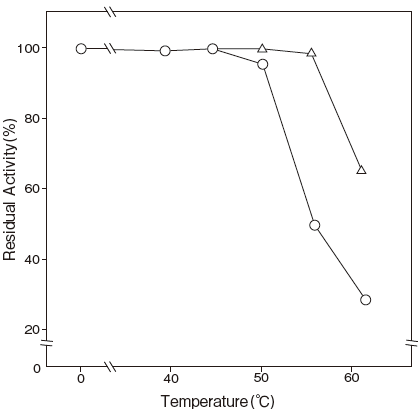
- Thermal stability
- : Stable at 50℃ and below
: (pH 7.5, 10 min) Figure 4
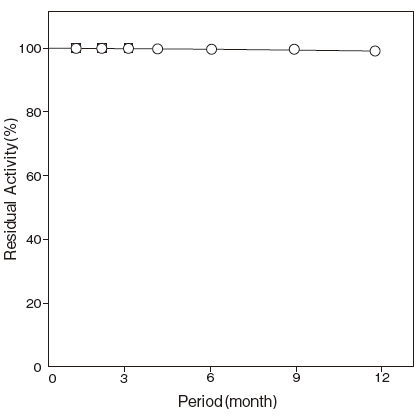
- Storage stability
Effects of various - : At least one year at −20℃Figure 5
- chemicals
- : See Table 2 and Table 3
- Stabilizer
- : ATP
- Activator
- : Triton X–100
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of fatty acid when coupled with Acyl–CoA oxidase (T–17) .
| ACS | ||
| FFA + CoA + ATP | → | Acyl - CoA + PPi + AMP |
| ACOD | ||
| Acyl - CoA + O2 | → | Enoyl - CoA + H2O2 |
| POD | ||
| 2 H2O2 + 4-AA + Phenol | → | Quinoneimine dye + 4 H2O |
FFA:Free fatty acid
Table 1. Substrate specificity (Fatty acids)
| Substrate | Relative
activity (%) |
Km value (10-5M) |
|---|---|---|
| Caproic acid (6:0) | 38 | |
| Caprylic acid (8:0) | 64 | 3.7 |
| Capric acid (10:0) | 24 | 0.5 |
| Lauric acid (12:0) | 21 | 0.95 |
| Myristic acid (14:0) | 40 | 0.71 |
| Palmitic acid (16:0) | 66 | 1.10 |
| Stearic acid (18:0) | 78 | 3.0 |
| Arachidic acid (20:0) | 100 | |
| Myristoleic acid (14:1) | 35 | |
| Palmitoleic acid (16:1) | 40 | |
| Palmitelaidic acid (16:1) | 48 | |
| Oleic acid (18:1) | 78 | 0.91 |
| Elaidic acid (18:1) | 60 | |
| Linoleic acid (18:2) | 57 | 0.34 |
| Linolenic acid (18:3) | 62 | 1.10 |
| Arachidonic acid (20:4) | 63 | |
| Erucic acid (22:1) | 92 | |
| Nervonic acid (24:1) | 9 |
Table 2. Effect of detergents on ACS activity
| Detergent (%) |
Relative activity (%) |
|
|---|---|---|
| None | 100 | |
| Deoxycholate | 0.1 | 69.6 |
| 0.25 | 41.1 | |
| SDS | 0.1 | 0 |
| Cetyltrimethyl ammoniumchloride | 0.1 | 96.4 |
| 0.25 | 0 | |
| Cetylpyridinium chloride | 0.1 | 96.4 |
| 0.25 | 0 | |
| Sarcosinate PN | 0.1 | 64.3 |
| 0.25 | 0 |
Table 2. Effect of metal ions on ACS activity
| Additive | Consentration | Relative activity (%) |
|---|---|---|
| None | - | 38 |
| KCI | 0.1M | 29 |
| NaCI | 0.1M | 35 |
| LiCl | 0.1M | 26 |
| NH4Cl | 0.1M | 29 |
| MgCl2 | 1mM | 100 |
| CaCl2 | 1mM | 94 |
| ZnCl2 | 1mM | 31 |
| BaCl2 | 1mM | 38 |
| MnCl2 | 1mM | 117 |
| CuCl2 | 1mM | 0 |
| NiCl2 | 1mM | 87 |
| EDTA | 1mM | 0 |
| 1mM MgCl2 + CaCl2 | 1mM | 103 |
| 1mM MgCl2 + ZnCl2 | 1mM | 55 |
| 1mM MgCl2 + CuCl2 | 1mM | 0 |
| 1mM MgCl2 + NiCl2 | 1mM | 99 |
| 1mM MgCl2 + BaCl2 | 1mM | 100 |
| 1mM MgCl2 + MnCl2 | 1mM | 117 |
Assay
Principle
The assay is based on the increase in absorbance at 550 nm as the formation of quinoneimine dye proceeds in the following reactions:
| ACS | ||
| Palmitic Acid+CoA+ATP | → | Palmitoyl CoA+AMP+PPi |
| ACOD | ||
| Palmitoyl CoA+O2 | → | 2–Hexadecenoyl–CoA+H2O2 |
ACOD : Acyl–CoA oxidase
MEHA : 3–Methyl–N–ethyl–N– (2–hydroxymethyl) aniline
Unit definition
One unit is defined as the amount of enzyme which converts 1 μmole of fatty acid to acyl–CoA per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture for the first reaction
0.2 M KH2PO4 –K2HPO4 buffer pH 7.5 0.20 ml 10 mM ATP solution pH 7.5 0.10 ml 10 mM MgCl2 solution 0.10 ml 1 mM Palmitic acid–5 % (W/V)
Triton X–100 solution pH 7.5 1)0.20 ml Distilled water 0.35 ml 10 mM CoA solution pH 6.5 2) 0.05 ml 1) : 1 mM Palmitic acid–5 % (W/V) Triton X–100
solution pH 7.5Dissolve 26 mg of palmitic acid with 90 ml of 5% (W/V)
Triton X–100, adjust pH to 7.5 at 25℃ with 4 N NaOH,
add 5% (W/V) Triton X–100 to make a total of 100 ml.2) : 10mM CoA solution pH 6.5
Dissolve 154 mg (purity calculation) of CoA with 15
ml of distilled water, adjust pH to 6.5 at 25℃ with 4 N
NaOH, and add distilled water to make a total of 20 ml
- Reaction mixture for the second reaction
0.2 M KH2PO4 –K2HPO4 buffer pH 7.5 0.50 ml 20mM NEM 0.10 ml 15mM 4–AA solution 0.30 ml 0.3% (W/V) MEHA solution pH 5.8 0.25 ml 100 U/ml POD solution 3) 0.10 ml 0.5% (W/V) NaN3 solution 0.10 ml Distilled water 0.55 ml 120 U/ml ACOD solution 4) 0.10 ml NEM : N–Ethylmaleimide 3) : 100 U/ml POD solution
Dissolve 1,000 U (PPU) of POD with 10 ml of distilled water.4) : 120 U/ml ACOD solution
Dissolve 1,200 U of ACOD with 10 ml of ACOD dilution buffer ※)※) : ACOD dilution buffer
Dissolve 1.36 g of KH2PO4 and 1.82 g of ATP with distilled water, adjust pH to 7.0 with 4 N NaOH, add 10 ml of 1 mM FAD, and finally add distilled water to make a total of 1 L. - Enzyme dilution buffer
10 mM KH2PO4 –K2HPO4 buffe (r pH 7.5) containing 2 mM ATP, 0.5% (W/V) BSA and 0.1 % (W/V) Triton X–100. - Reagents:
ATP (2Na・3H2O) : Kyowa Hakko Co., Ltd.
CoA (Coenzyme A) : KOHJIN
Palmitic acid :
FUJIFILM Wako Pure Chemical Corporation#169–00105Triton X–100: The Dow Chemical company
NEM: FUJIFILM Wako Pure Chemical Corporation
Special grade #058–020614–AA: NACALAI TESQUE, INC.
Special grade #01907–52MEHA: Tokyo Kasai Kogyo Co., Ltd. #E0220
POD: Sigma Chemical Co. Type Ⅱ #P–8250
ACOD: Asahi Kasei Pharma Corporation #T–17
FAD (2Na) : Kyowa Hakko Co., Ltd.
BSA: Millipore Fraction V pH 5.2 #81–053ATP : Adenosine triphosphate FAD : Flavine adenine dinucleotide
Enzyme solution
Weigh about 20 mg of test sample exactly and add enzyme dilution buffer to make a total of 20 ml. Dissolve it with enzyme dilution buffer to adjust the concentration to within 0.04–0.06 U/ml.
Procedure
- Pipette accurately 1.0 ml of reagent mixture for the first reaction into a small test tube and preincubate at 37℃.
- After 5 min, add exactly 50 μl of enzyme solution and mix to start the first reaction at 37℃.
※ In the case of a test blank, add 50 μl of enzyme dilution buffer in place of enzyme solution. - After 10 min, add 2.0 ml of reagent mixture for the second reaction to stop the first reaction and mix to start the second reaction at 37℃.
- After 5 min, measure the absorbance at 550 nm.
Absorbance sample : As blank : Ab △A = (As−Ab)≦0.30 Abs
Calculation
Activity (U/mg) = {(△ A/10)/(32.0 × 1/2)} × 3.05/0.05 × 1/x| 32.0 | millimolar extinction coefficient of quinoneimine dye at 550 nm (cm2 / μmole) |
| 1/2 : | a multiplier derived from the fact that 2 mole of H2O2 produces 1 mole of quinoneimine dye |
| 10 : | reaction time (min) |
| 3.05 : | final volume (ml) |
| X : | concentration of ACS in enzyme solution (mg/ml) |
Storage
Storage at −20℃ in the presence of a desiccant is recommended. Enzyme activity will be retained for at least one year under this condition.
References
- Yamada, H., Shimizu, S. and Tani, Y. (1980) Vitamin
(Japanese) , 54, 489. - Shimizu, S., Inoue, K., Tani, Y. and Yamada, H. (1980) Anal. Biochem., 107, 193.
- Okabe, H., Uji, Y., Nagashima, K. and Noma, A. (1980) Clin. Chem., 26, 1540.
- Shimizu, S., Inoue, K., Tani, Y. and Yamada, H. (1979) Anal. Biochem., 98, 341.
ACOD 活性測定法 (Japanese)
試薬液
- 第一反応試薬混合液
0.2M KH2PO4 –K2 HPO4 緩衝液pH7.5 0.20 ml 10mM ATP 溶液pH7.5 0.10 ml 10mM 塩化マグネシウム溶液 0.10 ml 1mM パルミチン酸–5% (W/V)
トリトンX–100 溶液pH7.5 1)0.20 ml 精製水 0.35 ml 10mM CoA 溶液pH6.5 2) 0.05 ml 1) : 1mM パルミチン酸–5% (W/V) トリトンX–100溶液pH7.5
パルミチン酸2 6 m g を5 % ( W / V ) トリトンX-100 溶液90ml で加温溶解した後、4N NaOHでpH7.5 (25℃) に調整し、5% (W/V) トリトンX–100 溶液で全容100ml とする。2) : 10mM CoA 溶液pH6.5
CoA 154mg (純度換算) を精製水15ml に溶解した後、4N NaOH でpH6.5 (25℃) に調整し、精製水で全容20ml とする。 - 第二反応試薬混合液
0.2M KH2PO4 –K2HPO4 緩衝液pH7.5 0.50 ml 20mM NEM 溶液 0.10 ml 15mM 4–AA 溶液 0.30 ml 0.3% (W/V) MEHA 溶液pH5.8 0.25 ml 100U/ml POD 溶液 3) 0.10 ml
-
0.5% (W/V) NaN3 溶液 0.10 ml 精製水 0.55 ml 120U/ml ACOD 溶液 4) 0.10 ml 3) : 100U/ml POD 溶液
POD 1,000 単位 (PPU) を精製水10ml で溶解する。4) : 120U/ml ACOD 溶液
ACOD 1,200 単位 (U) をACOD 希釈緩衝液※)
10ml で溶解する。※) : ACOD 希釈緩衝液
KH2PO4 1.36g とATP 1.82g を精製水に溶解した後、4N NaOH pH7.0 (25℃) に調製し、さらに1mM FAD 溶液10ml を加えて精製水で全容1L とする。 - 酵素溶解希釈用液
2mM ATPと0.5% (W/V) BSA 及び0.1% (W/V) トリトンX–100 を含む10mM KH2PO4 –K2HPO4 緩衝液pH7.5 - 試薬
ATP (アデノシン三リン酸・2Na・3H2O) :
協和発酵製CoA (コエンザイムA) :興人製
パルミチン酸:富士フイルム和光純薬製 特級
#169–00105トリトン (トリトンX–100) :Dow Chemical 製
NEM (N– エチルマレイミド) :
富士フイルム和光純薬製 特級 #058–020614–AA:ナカライテスク製 特級 #01907–52
- MEHA[N–エチル–N–2–ヒドロキシエチル–m– トルイジン]:
東京化成製 #E0220POD:シグマ製 Type Ⅱ #P–8250
ACOD (アシル–CoA 酸化酵素) :旭化成ファーマ製
#T–17FAD (フラビンアデニンジヌクレオチド・2Na) :
協和発酵製
BSA: Millipore 製 Fraction V pH5.2 #81–053
酵素試料液
- 検品約20mg を精密に量り、酵素溶解希釈用液に溶解して全容20ml とする。 その液を酵素溶解希釈用液で0.04~0.06U/ml 濃度と なるように適宜希釈する。
測定操作法
- 小試験管に第一反応試薬混合液1.0ml を正確に分注して37℃で予備加温する。
- 5 分経過後、酵素試料液50 μl を加えて混和し、37℃で反応を開始する。
※ 盲検は酵素試料液の代わりに酵素溶解希釈用液
50 μ1 を加える。
- 10 分経過後、第二反応試薬混合液2.0ml を正確に加えて混和し、第一反応を停止させ、37℃で第二反応を開始する。
- 5 分経過後、550nm における吸光度を測定する。求められた吸光度を試料液はAs、盲検液はAb とする。
ΔA= (As−Ab) ≦ 0.30 Abs
計算
活性 (U/mg) = {(△ A/10)/(32.0 × 1/2)} × 3.05/0.05 × 1/x| 32.0 : | キノンイミン色素の550nm におけるミリモル 分子吸光係数 (cm2 / μmole) |
| 1/2 : | H2O2 2 モルからキノンイミン色素1 モルが生成す ることによる係数 |
| 10 : | 反応時間 (min) |
| 3.05 : | 反応総液量 (ml) |
| 0.05 : | 反応に供した酵素試料液量 (ml) |
| X : | 酵素試料液中の検品濃度 (mg/ml) |