Preparation and Specification
- Appearance
- : Colorless to brownish solution
- Specific activity
- : More than 200 U/ml
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 15 kDa (Sephadex G–100)
- Isoelectric point
- : pH 7.51
- Michaelis constants
- : Lecithin 5.0 × 10–3M
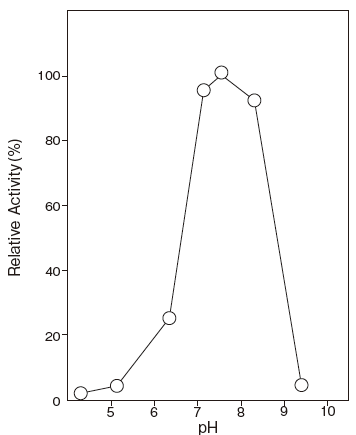
- Optimum pH
- : 7.3–8.3Figure 1
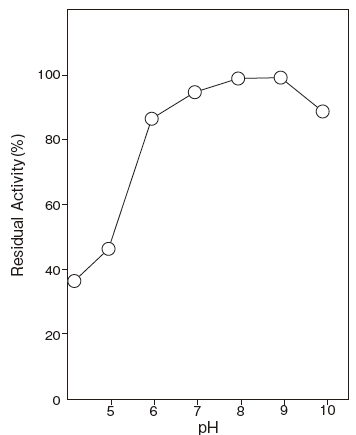
- pH stability
- : 6.0–9.5 (55℃, 10 min) Figure 2
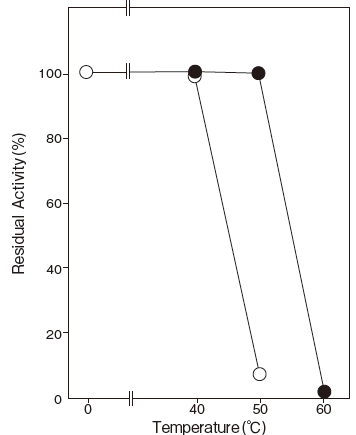
- Thermal stability
- : Stable at 50℃ and below (pH8.0, 10 min, + CaCl2) Figure3
- Stabilizer
- : Ca2+
- Activator
- : Ca2+, Non–ionic detergents
- Inhibitor
- : EDTA
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of lecithin when coupled with lysophospholipase (T–32) , glycerophosphorylcholine phosphodiesterase (T–33) and choline oxidase (T–05) .
| PLA2ⅡL | ||
| Lecithin + H2O | → | Lysolecithin + Fatty acid |
| LYPL | ||
| Lysolecithin + H2O | → | Glycerophosphorylcholine + Fatty acid |
| GPCP | ||
| Glycerophosphorylcholine + H2O | → | Glycerophosphate + Choline |
| COD | ||
| Choline + 2 O2 + H2O | → | Betaine + 2 H2O2 |
| POD | ||
| 2 H2O2 + 4-AA + Phenol | → | Quinoneimine dye + 4 H2O |
Table 1. Substrate specificity
| Substrate | Relative activity (%) |
|---|---|
| Phosphatidylcholine | 100 |
| Phosphatidylethanolamine | 30 |
| Phosphatidic Acid | 10 |
| Lysophosphatidylcholine | 0 |
| Triolein | 0 |
| Trilaurin | 0 |
Assay
Principle
-
The assay is based on the increase in absorbance at 500 nm as the formation of quinoneimine dye proceeds in the following reactions:
| PLA2ⅡL | ||
| Lecithin+H2O | → | Lysolecithin+Fatty acid |
| ACS | ||
| Fatty acid+CoA+ATP | → | Acyl–CoA+AMP+PPi |
| ACOD | ||
| Acyl–CoA+O | → | 2, 3–Enoyl acyl–CoA+H2O2 |
| POD | ||
| 2 H2O2+4–AA+Phenol | → | Quinoneimine dye+4 H2O |
ACS:Acyl–CoA synthetase
ACOD: Acyl–CoA oxidase
ATP: Adenosine triphosphate
Unit definition
-
One unit is defined as the amount of enzyme which liberates 1 μmole of fatty acid per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture for the first reaction
0.2 M Tris–HCl buffer pH 8.0 0.20 ml 20 mM DPPC solution 1) 0.05 ml 1 M CaCl2 solution 0.025 ml 0.2 M ATP solution pH 7.0 0.01 ml 0.1 M CoA solution pH 6.0 2) 0.02 ml 10.6 U/ml ACS solution 3) 0.05 ml
-
Distilled water 0.145 ml 1) : 20 mM DPPC solution
Dissolve 147 mg of DPPC with 10 ml of 2% (W/V)
Triton X–100 solution.2) : 0.1 M CoA solution pH 6.0
Dissolve 7.85 g (purity calculation) of CoA with 80 ml of distilled water and adjust pH to 6.0 (25℃) with 4 N NaOH and add distilled water to make a total of 100ml.3) : 10.6 U/ml ACS solution
Dissolve 106 U of ACS with 10 ml of Tris–HCl buffer pH 8.0.DDPC: Dipalmytoylphosphatidylcholine - Reaction mixture for the second reaction
0.5 M PIPES–NaOH buffer pH 7.5 0.04ml 0.3% (W/V) 4–AA solution 0.10ml 0.2% Phenol solution 0.10ml 50 U/ml POD solution4) 0.045ml 300 U/ml ACOD solution5) 0.05ml 10% (W/V) Triton X–100 solution 0.01ml 0.2 M ATP solution pH 7.0 0.05ml 10 mM FAD solution 0.005ml Distilled water 0.10ml 4) : 50 U/ml POD solution
Dissolve 500 U (PPU) of POD with 10 ml of distilled water.5) : 300 U/ml ACOD solution
Dissolve 3,000 U of ACOD with 10 ml of PIPES–NaOH buffer pH 7.5.FAD: Flavine adenine dinucleotide
- 20 mM NEM
NEM: N–ethylmaleimide - Reaction stopper
0.1 M EDTA solution containing 0.5% (W/V) SDS pH8.0SDS: Sodium dodecyl sulfate EDTA: Ethylenediamine tetraacetic acid - Enzyme dilution buffer
10 mM Tris–HCl buffer pH 8.0 containing 0.05% (W/V) BSA. - Reagents:
DPPC: Nippon Fine Chemical Co., Ltd.
Coenzyme A (CoA・Li3) : Kohjin Co.
ATP (2Na・3H2O) : Kyowa Hakko Co., Ltd.
PIPES[Piparazine–1,4–bis (2–ethanesulphonic acid) ]:
Dojindo Laboratories #345–02225Triton X–100: The Dow Chemical Company
NEM: FUJIFILM Wako Pure Chemical Corporation
#058–02061ACS: Asahi Kasei Pharma Corporation #T–16
ACOD: Asahi Kasei Pharma Corporation #T–17
SDS: NACALAI TESQUE, INC. #316–06
4–AA: NACALAI TESQUE, INC. Special grade
#01907–52POD: Sigma Chemical Co. Type Ⅱ #P–8250
EDTA (2Na・2H2O) : KISHIDA CHEMICAL Co., Ltd.
#060–29133
Enzyme solution
-
Dilute accurately 0.5 ml of the sample with enzyme dilution buffer to make a 50-fold Solution. Dilute it with enzyme dilution buffer to adjust the concentration as required.
Procedure
- Pipette accurately 0.50 ml of reaction mixture for the first reaction into a small test tube and preincubate at 37℃.
- After 5 min, add exactly 50 μl of enzyme sample and mix to start the first reaction at 37℃.
※ In the case of a test blank, add 50 μ1 of enzyme dilution buffer in place of enzyme solution at this point.
- After 10 min, add 0.5 ml of 20 mM NEM and add 0.5ml of reaction mixture for the second reaction after 15 seconds later and mix to start the second reaction at 37℃.
- At 5 min after starting the reaction, add 1.5 ml of the reaction stopper to stop the reaction.
- Measure the absorbance at 500 nm.
△A = (As−Ab) ≦ 0.20 AbsAbsorbance sample : As blank : Ab
Calculation
- Activity (U/mg) = {(△A/10)/(12.0×1/2)} × 3.05/0.05 × D
-
12.0 : millimolar extinction coefficient of quinoneimine dye at 500 nm (cm2/μmole)1/2 : a multiplier derived from the fact that 2 mole of H2O2 produces 1 mole of quinoeimine dye 10 : reaction time (min) 3.05 : final volume (ml) 0.05 : volume of enzyme solution (ml) D : times of dilution in enzyme solution
Storage
-
Storage at 2~8℃ in the presence of a desiccant is recommended.
References
- Scandella, C. J. and Kornberg, A. C. (1971) Biochemistry, 10, 4447–4456.
- Nishijima, M., Akamatsu, Y. and Nojima, S. (1974) J. Biol. Chem., 249, 5658–5667.
- Waite, M. and Sisson, P. (1971) Biochemistry, 10, 2377–2383.
- Kannagi, R. and Koizumi, K. (1979) Biochim. Biophys.
Acta, 556, 423–433. - Van Dam–Mieras, M. C. E., Slotboom, A. J., Pieterson,
W. A. and de Haas, G. H. (1975) Biochemistry, 14, 5387–5393.
PLA2ⅡL活性測定法 (Japanese)
試薬液
- 第一反応試薬混合液
0.2M トリス–HCl 緩衝液 pH8.0 0.20 ml 20mM DPPC 溶液1) 0.05 ml 1M 塩化カルシウム溶液 0.025 ml 0.2M ATP 溶液 pH7.0 0.01 ml 0.1M CoA 溶液 pH6.02) 0.02 ml 10.6U/ml ACS 溶液3) 0.05 ml 精製水 0.145 ml 1) : 20mM DPPC 溶液
DPPC 147mg を2% (W/V) トリトンX–100 溶液10ml で溶解する。
-
2) : 0.1M CoA 溶液 pH6.0
CoA 7.85g (純度換算) を精製水80ml に溶解した後、4N NaOH でpH6.0 (25℃) に調整し、精製水で全容100ml とする。3) : 10.6U/ml ACS 溶液
ACS 106 単位 (U) を10mM トリス−HCl 緩衝液pH8.0 10ml で溶解する。 - 第二反応試薬混合液
0.5M PIPES–NaOH 緩衝液 pH7.5 0.04 ml 0.3% (W/V) 4–AA 溶液 0.10 ml 0.2% (W/V) フェノール液 0.10 ml 50U/ml POD 溶液4) 0.045 ml 300U/ml ACOD 溶液5) 0.05 ml 10% (W/V) トリトンX–100 溶液 0.01 ml
-
0.2M ATP 溶液 pH7.0 0.05 ml 10mM FAD 溶液 0.005 ml 精製水 0.10 ml 4) : 50U/ml POD 溶液
POD 500 単位 (PPU) を精製水10ml で溶解する。5) : 300U/ml ACOD 溶液
ACOD 3,000 単位 (U) を10mM PIPES–NaOH
緩衝液pH7.5 10ml で溶解する。 - 20mM NEM 溶液
- 反応停止液
0.5% (W/V) SDS を含む0.1M EDTA 溶液 pH8.0 - 酵素溶解希釈用液
0.05% (W/V) BSA を含む10mM トリス−HCl 緩衝液pH8.0 - 試薬
DPPC (ジパルミトイルフォスファチジルコリン):日本精化製CoA (コエンザイム・Li3) :興人製
ATP (アデノシン三リン酸・2Na・3H2O) :協和発酵製PIPES[ピペラジン–1,4– ビス (2– エタンスルホン酸) ]:
同仁化学製 #345–02225トリトンX–100:Dow chemical 製
FAD (フラビンアデニンジヌクレオチド・2Na) :協和発酵製NEM (N– エチルマレイミド) :
富士フイルム和光純薬製 特級 #058–02061ACS:旭化成ファーマ製 #T–16
ACOD:旭化成ファーマ製 #T–17
SDS (ドデシル硫酸ナトリウム) :ナカライテスク製 #316–064–AA:ナカライテスク製 特級 #01907–52
POD: シグマ製 Type Ⅱ #P–8250
EDTA (エチレンジアミン四酢酸・2Na・2H2O) :キシダ化学製 #060–29133
酵素試料液
- 検品0.5ml を酵素溶解希釈用液で50 倍に希釈する。
- その液を酵素溶解希釈用液で適宜希釈する。
測定操作法
- 小試験管に第一反応試薬混合液0.50ml を正確に分注し37℃で予備加温する。
- 5 分経過後、酵素試料液50 μl を正確に加えて混和し、37℃で第一反応を開始する。
※ 盲検は酵素試料液の代わりに酵素溶解希釈緩衝液50 μl を加える。 - 10 分経過後、20mM NEM 溶液0.50ml を加えて混和し、15 秒後に第二反応試薬混合液0.50ml を加えて混和し、37℃で第二反応を開始する。
- 5 分経過後、反応停止液1.50ml を加えて混和し、反応を停止する。
- 500nm における吸光度を測定する。
求められた吸光度を試料液はAs、盲検液はAb とする。
ΔA = (As−Ab) ≦ 0.20 Abs.
計算
活性 (U/mg) = {(△A/10)/(12.0×1/2)} × 3.05/0.05 × D| 12.0 : | キノンイミン色素の500nm におけるミリモル分子吸光係数 (cm2 / μmole) |
| 1/2 : | H2O2 2 モルからキノンイミン色素1 モルが生成することによる係数 |
| 10 : | 反応時間 (min) |
| 3.05 : | 反応総液量 (ml) |
| 0.05 : | 反応に供した酵素試料液量 (ml) |
| D : | 酵素試料液の調製希釈倍数 |