DIAPHORASE (NADH) [DIⅡ]
from Bacillus megaterium
(NADH: acceptor oxidoreductase, EC 1.6.5.2)
NADH + H+ + A → NAD+ + AH2
A : Hydrogen acceptor
Preparation and Specification
- Appearance
- : Yellow to yellow brownish lyophilized powder
- Specific activity
- : More than 70 U/mg solid
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 50 kDa ( SDS-PAGE) , 160 kDa (gel filtration)
- Isoelectric point
- : pH 4.2
- Michaelis constants
- : NADH 5.5 × 10-4M
- Optimum pH
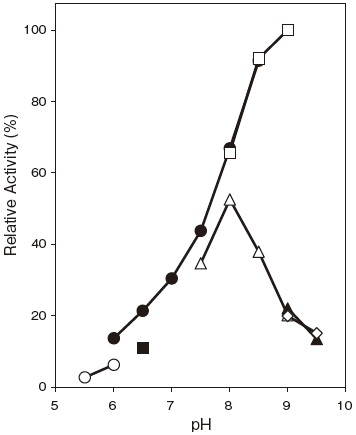
- : 8.0–9.0Figure 1
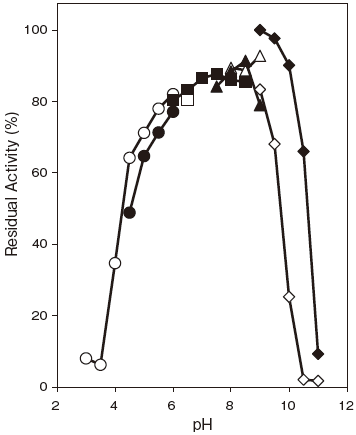
- pH stability
- : 6.0–9.0 (37℃, 60 min) Figure 2
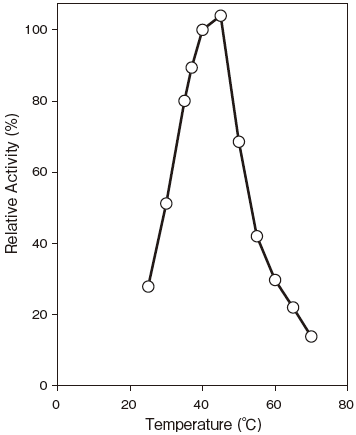
- Optimum temperatute
- : Optimum temperatuteFigure3
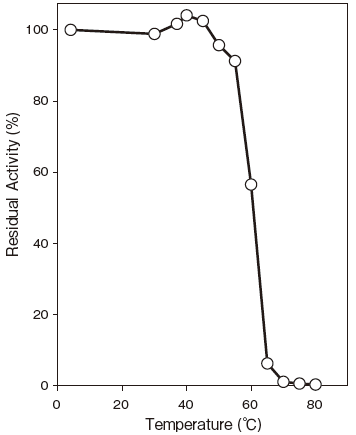
- Thermal stability
- : Stable at 50℃ and below (pH 8.0, 10 min) Figure4
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of reduced NAD.
| DIⅡ | ||
| NADH + H+ + NTB | → | Formazane dye + NAD+ |
Table 1. Substrate specificity
| Substrate | Relative activity (%) |
|---|---|
| NADH |
100 |
| NADPH |
16 |
Assay
Principle
-
The assay is based on the increase in absorbance at 550 nm as the formation of formazane dye (NTBH2) proceeds in the following reaction:
| DI Ⅱ | ||
| NADH+H++NTB | → | NAD++Formazane dye |
NADH:Nicotineamido adenine dinucleotide
NTB:Nitrotetrazolium blue
Unit definition
-
One unit is defined as the amount of enzyme which oxidizes 1 μmole of NADH to NAD+ per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture
0.2 M KH2PO4–NaOH buffer
pH 8.00.50 ml 0.25% (W/V) NTB solution 0.10 ml 1% (W/V) BSA solution 0.10 ml 10 mM NADH solution 0.10 ml Distilled water 0.20 ml - Reaction stopper
0.1 N HCl solution - Enzyme dilution buffer
0.1 M KH2PO4–NaOH buffer pH8.0 containing 0.1%(w/v) BSA - Reagents
NTB: Dojindo Laboratories # 344–02033
BSA: Millipore Fraction V pH5.2 #81–053
NADH (2Na・3H2O・reduced form) :Kyowa Hakko Co. Ltd.
Enzyme solution
- Accurately weigh about 20 mg of the sample and add enzyme dilution buffer to make a total of 20 ml. Dilute it with enzyme dilution buffer to adjust the concentration as required.
Procedure
- Pipette accurately 1.0 ml of reaction mixture into a small test tube and preincubate at 37℃.
- After 5 min, add exactly 100 μl of enzyme solution and mix to start the reaction at 37℃.
※ In the case of a test blank, add 100 μl of enzyme dilution buffer in place of enzyme solution. - At 10 min after starting the reaction, add 2.0 ml of the reaction stopper to stop the reaction.
- Measure the absorbance at 550 nm.
△ A = (As−Ab) ≦ 0.370 AbsAbsorbance sample : As blank : Ab
Calculation
- Activity (U/mg of powder) = {(△A/10)/12.4} × 3.10/0.10 × 1/x
12.4 : millimolar extinction coefficient of Formazane dye at 550 nm (cm2/ μmole)10 : reaction time (min) 3.10 : final volume (ml) 0.10 : volume of enzyme solution (ml) X : concentration of the sample in enzyme solution (mg/ml)
Storage
-
Storage at − 20℃ in the presence of a desiccant is recommended. Enzyme activity will be retained for at least one year under this condition.
References
- Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, R. J. (1951) J. Biol. Chem., 193, 265–275.
- Gerlo, E. and Charlier, J. (1975) Eur. J. Biochem., 57, 40–467.
- Jablonski, E. and DeLuca, M. (1977) Biochemistry, 16, 2932–2936.
- Watanabe, H. and Hastings, J. W. (1982) Mol. Cell.
Biochem., 44, 181–187.
DI Ⅱ活性測定法 (Japanese)
試薬液
- 反応試薬混合液
0.2M KH2PO4–NaOH 緩衝液pH8.0 0.50 ml 0.25% (W/V) NTB 溶液 0.10 ml 1% (W/V) BSA 溶液 0.10 ml 10mM NADH 溶液 0.10 ml 精製水 0.20 ml - 反応停止液
0.1N HCl 液 - 酵素溶解希釈用液
0.1% (W/V) BSA を含む0.1M KH2PO4–NaOH 緩衝液 pH8.0 - 試薬
NTB (ニトロテトラゾリウムブルー) :同仁化学製 #344–02033
BSA: Millipore 製 Fraction V pH5.2 #81–053
NADH (2Na・3H2O・還元型) :協和発酵製
酵素試料液
- 検品約20mg を精密に量り、酵素溶解希釈用液に溶解して全容20ml とする。
その液を酵素溶解希釈用液で適宜希釈する。
測定操作法
- 小試験管に反応試薬混合液1.0ml を正確に分注し、37℃で予備加温する。
- 5 分経過後、酵素試料液100 μl を正確に加えて混和し、37℃で反応を開始する。
※ 盲検は酵素試料液の代わりに酵素溶解希釈用液100 μl を加える。 - 10 分経過後、反応停止液2.0ml を正確に加えて混和し、反応を停止させる。
- 550nm における吸光度を測定する。
求められた吸光度を試料液はAs、盲検液はAb とする。
ΔA = (As-Ab) ≦ 0.370 Abs
計算
活性 (U/mg) = {(△ A/10)/12.4} × 3.10/0.10 × 1/x| 12.4 : | NTBH2 の550nm におけるミリモル分子吸光係数 (cm2/ μmole) |
| 10 : | 反応時間 (min) |
| 3.10 : | 反応総液量 (ml) |
| 0.10 : | 反応に供した酵素試料液量 (ml) |
| X : | 酵素試料液中の検品濃度 (mg/ml) |
Copyright © Asahi Kasei Pharma Corporation. All rights reserved.