CHOLESTEROL ESTERASE [CEBPII]
from Microorganism
(Sterol‒ester acylhydrolase, EC 3.1.1.13)
(Sterol esterase)
Cholesterol Ester + H2O → Cholesterol + Fatty Acid
High liquid stability
Preparation and Specification
- Appearance
- : White to off white lyophilized powder
- Specific activity
- : More than 10.0 U/mg solid
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 63 kDa (SDS‒PAGE)
- Isoelectric point
- : 5.1 (estimated from amino acid sequence)
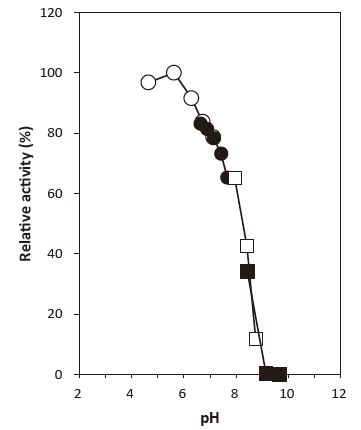
- Optimum pH
- : 5.6Figure 1
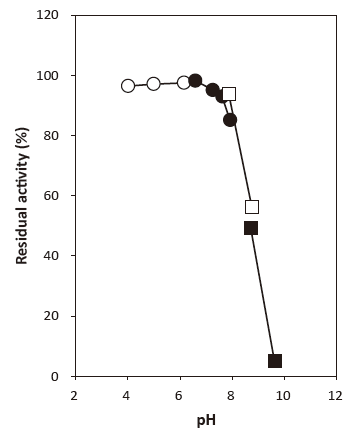
- pH stability
- : 4.0‒8.0 (37°C, 60 min)Figure 2
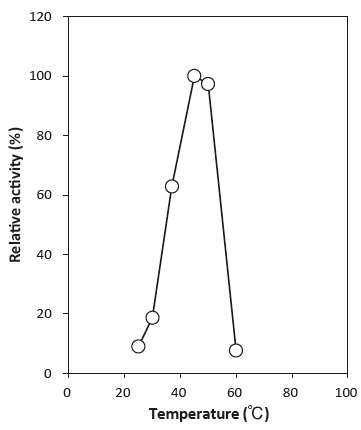
- Optimum temperature
- : 45℃Figure 3
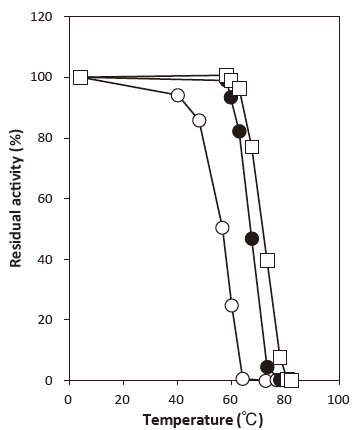
- Thermal stability
- : Stable at 45°C and below (pH6.0, 30 min) Figure 4
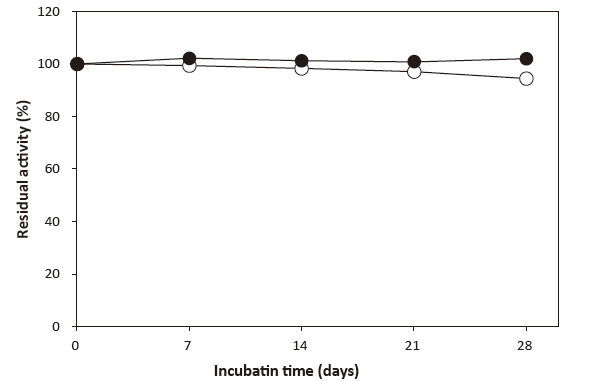
- Liquid stability
- : See Figure 5
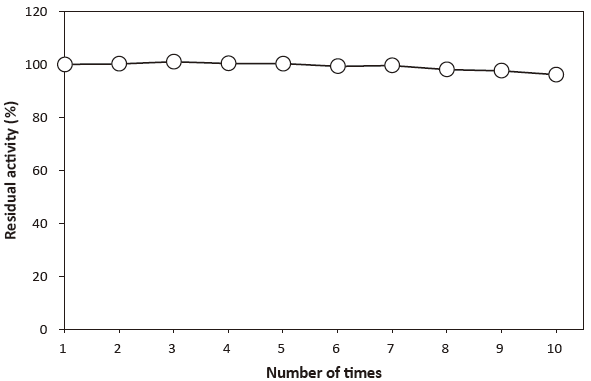
- Adsorption on glass
- : See Figure 6
- Effect of coexisting ions
- : See Table 2
- Effect of detergents
- : See Table 3
- Light stability
- : See Table 4
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of total cholesterol, HDL-C, and LDL-C coupled with cholestreol oxidase (T‒84 and T‒101) .
This enzyme is suitable for assembling in liquid reagens.
| CEBPII | ||
| Cholesterol ester + H2O | → | Cholestero + FFA |
| CON Ⅱ | ||
| Cholesterol + O2 | → | △4 -Cholesten-3-one + H2O2 |
| POD | ||
| 2 H2O2 + 4-AA + Phenol | → | Quinoneimine dye + 4 H2O |
FFA: Free fatty acid
Table 1 Substrate specificity
| Substrate (0.95mM) | Relative activity (%) | |
|---|---|---|
| Cholesterol Acetate | C 2:0 | 0.4 |
| Cholesterol Propionate | C 3:0 | 9.3 |
| Cholesterol Butyrate | C 4:0 | 27.8 |
| Cholesterol Palmitate | C16:0 | 19.8 |
| Cholesterol Stearate | C18:0 | 2.0 |
| Cholesterol Oleate | C18:1 | 100.0 |
| Cholesterol Linoleate | C18:2 | 80.6 |
Table 2 Effect of coexisting ions on CEBPⅡ activity
| Coexisting ion (20 mM) | Relative activity (%) |
|---|---|
| None | 100 |
| NaCl | 101 |
| KCl | 100 |
| NH4Cl | 100 |
| CaCl2 | 101 |
| MgCl2 | 101 |
| MnCl2 | 103 |
| ZnCl2 | 105 |
| EDTA | 102 |
| NaN3 | 100 |
| NaF | 98 |
All samples contain 0.1% BSA
Table 3 Effect of detergents on CEBP II activity
| Detergent (0.1%) | Relative activity (%) |
|---|---|
| None | 100 |
| Triton X-100 | 115 |
| Adekatol TN-100 | 117 |
| Adekatol SO-120 | 142 |
| Newcol-707 | 115 |
| Newcol-710 | 113 |
| Emulgen 120 | 141 |
| Emulgen 705 | 135 |
| Deoxycholic acid | 129 |
| Tauroursodeoxycholic acid | 134 |
| Sodium dodecyl sulfate | 127 |
Table 4 Light stability
| Form of CEBP II | Light source | Residual activity (%) |
|---|---|---|
| Powder | LED | 96.5 |
| Fluorescent | 98.7 | |
| Solution | LED | 94.1 |
| (1mg/ml, water) | Fluorescent | 98.4 |
After 24 hours of incubation
Assay
Principle
-
The assay is based on the increase in absorbance at 493 nm as the formation of quinoneimine dye proceeds in the following reactions:
| CEBPⅡ | ||
| Cholesterol ester+H2O | → | Cholesterol+Fatty acid |
| CO | ||
| Cholesterol+O2 | → | △4–Cholesten–3–one+H2O2 |
| POD | ||
| 2 H2O2+4–AA+Phenol | → | Quinoneimine dye+4 H2O |
CO: Cholesterol oxidase
Unit definition
-
One unit is defined as the amount of enzyme which liberates 1 μmole of cholesterol per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture
0.2M KH2PO4‒NaOH buffer pH 6.8 0.60 ml 0.35% 4‒AA solution 0.30 ml 0.2% (W/V) Phenol solution 0.30 ml 100U/ml POD solution1) 0.30 ml 3% (W/V) Triton X‒100 solution 0.30 ml 0.2U/ml CONⅡ solution2) 0.60 ml Substrate solution3) 0.30 ml Distilled water 0.30 ml 1) : 100U/ml POD solution
Dissolve 1000 U (PPU) of POD with 10 ml of distilled water.2) : 0.2U/ml CONⅡ solution
Dissolve 2 U of CONⅡ with CONⅡ dilution buffer※)
※) : CONⅡ dilution buffer
0.1M KH2PO4‒Na2HPO4 buffer pH 7.0 containing
0.05% (W/V) Triton X‒100.3) : Substrate solution
Calf serum - Enzyme dilution buffer
10mM KH2PO4‒NaOH buffer pH 7.5 containing 0.1%
(W/V) BSA. - Reagents
Triton X‒100: The Dow Chemical Company
CONⅡ : Asahi Kasei Pharma Corporation #T‒84
Calf serum: GIBCO Co. (USA)
BSA: Millipore Fraction V pH5.2 #81‒053
4‒AA: NACALAI TESQUE, INC. Special grade
#01907‒52POD: Sigma Chemical Co. Type Ⅱ #P‒8250
Enzyme solution
-
Accurately weigh about 20 mg of the sample and add enzyme dilution buffer to make a total of 20 ml. Dilute it with enzyme dilution buffer to adjust the concentration to within 0.35 U/ml.
Procedure
- Pipette accurately 3.0 ml of reaction mixture into a small test tube and preincubate it at 37℃.
- After 10 min, add 50 μl of enzyme solution and mix to
start the reaction at 37℃.※ In the case of a test blank, add 50 μl of enzyme dilution buffer in place of enzyme solution. - After starting the reaction, measure the rate of increase per minute in absorbance at 493 nm. The rate must be measured within the linear portion of the absorbance
curve. -
△A/min = (As/min-Ab/min) ≦0.040 Abs/minAbsorbance sample : As/min blank : Ab/min
Calculation
-
Activity (U/mg of powder) = {(△A/min)/(12.0 × 1/2)} × 3.05/0.05 × 1/x
12.0 : millimolar extinction coefficient of quinoneimine dye
at 493 nm (cm2/ μmole)1/2 : a multiplier derived from the fact that 2 mole of
H2O2 produce 1 mole of quinoneimine dye3.05 : final volume (ml) 0.05 : volume of enzyme solution (ml) X : concentration of the sample in enzyme solution ( mg/ml)
Storage
-
Storage at -20℃ in the presence of a desiccant is recommended.
References
- Bradford, M. B., (1976) Anal. Biochem., 72, 248‒254.
- Allain, C. C., Poon, L. S., Chan, C. S. G., Richmond, W. and Fu, O.C. (1974) Clin. Chem., 20, 470‒475.
- Kameno, Y., Nakano, N. and Baba, S. (1976) Jap. J. Clin. Path., 24, 650.
CEBPⅡ 活性測定法 (Japanese)
試薬液
- 反応試薬混合液
0.2M2 KHPO4‒NaOH 緩衝液 pH6.8 0.60 ml 0.35% 4‒AA 溶液 0.30 ml 0.2% (W/V) フェノール溶液 0.30 ml 100U/ml POD 溶液 1) 0.30 ml 3% (W/V) トリトンX‒100 溶液 0.30 ml 0.2U/ml CONⅡ溶液 2) 0.60 ml 基質溶液 3) 0.30 ml 精製水 0.30 ml 1) : 100U/ml POD 溶液
POD1,000 単位 (PPU) を精製水10ml で溶解する。2) : 0.2U/ml CONⅡ溶液
CONⅡ 2 単位 (U) をCONⅡ溶解用液※) 10ml で溶解する。※) : CONⅡ溶解用液
0.05% (W/V) トリトンX‒100 を含む0.1M
KH2PO4‒Na2HPO4 緩衝液 pH7.03) : 基質溶液
仔牛血清液 - 酵素溶解希釈用液
0.1% (W/V) BSA を含む10mM KH2PO4‒NaOH 緩衝液 pH7.5 - 試薬
トリトンX‒100:Dow Chemical 社製
CONⅡ (コレステロール酸化酵素) :旭化成ファーマ製 #T‒84仔牛血清液 (Calf serum) : GIBCO (USA) 製
BSA: Millipore 製 Fraction V pH5.2 #81‒053
POD:シグマ製 Type Ⅱ #P‒8250
4‒AA:ナカライテスク製 特級 #01907‒52
酵素試料液
- 検品約20mg を精密に量り、酵素溶解希釈用液に溶解して全容20ml とする。
その液を酵素溶解希釈用液で約0.35U/ml 濃度となるように適宜希釈する。
測定操作法
- 小試験管に反応試薬混合液を3.0ml 正確に分注して37℃で予備加温する。
- 10 分経過後、酵素試料液50 μl を正確に加えて混和し、37℃で反応を開始する。
※ 盲検は酵素試料液の代わりに酵素溶解希釈用液50μl を加える。 - 反応開始後、493nm における吸光度を測定して直線的に反応している1 分間当たりの吸光度変化を求める。
求められた吸光度変化を試料液はAs/min、盲検液はAb/min とする。
Δ A/min = (As/min-Ab/min) ≦ 0.040Abs/min
計算
活性 (U/mg)= {(ΔA/min)/(12.0 × 1/2)} × 3.05/0.05 × 1/x| 12.0 : | キノンイミン色素の493nm におけるミリモル分子吸光係数 (cm2 /μmole) |
| 1/2 : | H2O22 モルからキノンイミン色素1 モルが生成することによる係数 |
| 3.05 : | 反応総液量 (ml) |
| 0.05 : | 反応に供した酵素試料液量 (ml) |
| X : | 酵素試料液中の検品濃度 (mg/ml) |