PYRUVATE OXIDASE [POPG]
from Aerococcus viridans
(Pyruvate: oxygen oxidoreductase, phosphorylating, EC 1.2.3.3)
Pyruvate + Pi + O2 → Acetylphosphate + CO2 + H2O2
Preparation and Specification
- Appearance
- : Yellowish amorphous powder, lyophilized
- Specific activity
- : More than 25 U/mg solid
- Contaminants
- :
- Lactate oxidase
- Less than 0.002 % (U/U)
- Total AST (GOT)
- Less than 0.002 % (U/U)
- Total ALT (GPT)
- Less than 0.006 % (U/U)
- Catalase
- Less than 0.3 % (U/U)
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 155 kDa (gel filtration)
70 kDa (SDS–PAGE)
- Isoelectric point
- : pH 4.0
- Michaelis constants
- : Pyruvate 5.9 × 10-3M (Mn2+)
Pyruvate 4.1 × 10-2M (Mg2+)
Phosphate 2.0 × 10-3M (Mn2+)
Phosphate 4.5 × 10-3M (Mg2+)
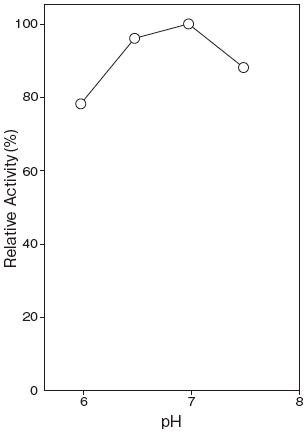
- Optimum pH
- : 6.5–7.0Figure 1
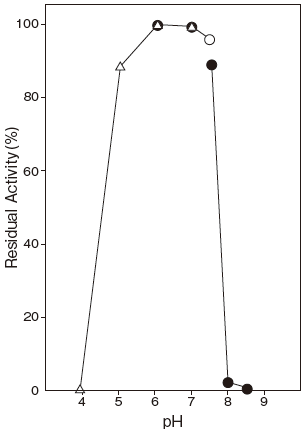
- pH stability
- : 6.0–7.0 (37℃, 60 min, 10 μM FAD) Figure 2
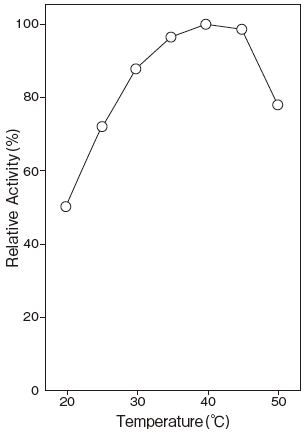
- Optimum temperature
- : 40℃Figure 3
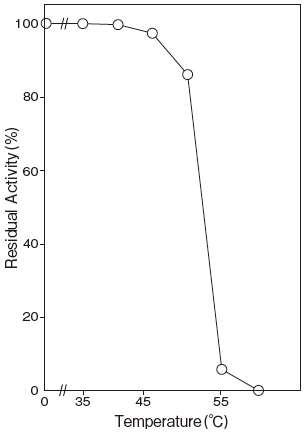
- Thermal stability
- : Stable at 45℃ and below (phosphate buffer containing 10 μM FAD, pH 6.5, 10 min) Figure 4
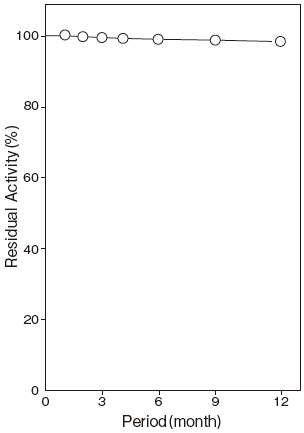
- Storage stability
- : At least one year at −20℃Figure 5
- Stabilizer
- : FAD
- Activators
- : Mn2+, Mg2+, Ca2+, Co2+
- Inhibitors
- : EDTA
- Co–factors
- : FAD, TPP
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of AST and ALT when coupled with oxaloacetate decarboxylase (T–209) (in the case of AST) and POD.
| ALT: α-KG + L-Alanine | → | L-Glutamate + Pyruvate |
| AST: α-KG + L-Aspartate | → | L-Glutamate + Oxaloacetate |
| OACⅡ | ||
| Oxaloacetate | → | Pyruvate + CO2 |
| POPG | ||
| Pyruvate + Pi + O2 | → | Acetylphosphate + CO2+ H2O2 |
| POD | ||
| 2 H2O2 + 4-AA + Phenol | → | Quinoneimine dye + 4 H2O |
Table 1. Substrate specificity
| Substrate | Relative activity (%) |
|---|---|
| Pyruvate | 100 |
| 2–Oxobutyrate | 3 |
| 2–Oxoglutarate | 0 |
| Oxaloacetate | 0 |
| DL–Lactate | 0 |
| Acetate | 0 |
| L–Alanine | 0 |
| L–Aspartate | 0 |
Assay
Principle
The assay is based on the increase in absorbance at 565nm as the formation of quinoneimine dye proceeds in the following reactions:
| POPG | ||
| Pyruvate+O2+Pi | → | Acethylphosphate+CO2+H2O2 |
| POD | ||
| 2 H2O2+4–AA+DMA+H + | → | Quinoneimine dye+4 H2O |
DMA: N, N–Dimethylaniline
Unit definition
-
One unit is defined as the amount of enzyme which generates 1μmole of H2O2 per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture Ⅰ
1 M KH2PO4 –NaOH buffer pH 6.7 0.20 ml 10 mM Thiamine pyrophosphate 0.02 ml 1 mM FAD solution 0.01 ml 100 U/ml POD solution 1) 0.05 ml 15 mM 4–AA solution 0.10 ml Distilled water 0.22 ml FAD: Flavine adenine dinucleotide 1) : 100 U/ml POD solution
-
Dissolve 1,000 U (PPU) of POD with 10 ml of distilled water. - Reaction mixture Ⅱ
0.2 % (V/V) DMA soultion 0.20 ml 0.1 M MgCl2 solution 0.10 ml - Substrate solution
1 M Potassium pyruvate solution - Reaction stopper
McIlvaine buffer pH 5.50–5.55 containing 0.1 M EDTAEDTA: Ethylenediamine tetraacetic acid - Enzyme dilution buffer
10 mM KH2PO4–NaOH buffer pH 7.0 containing
10 μM FAD - Reagents
Thiamine pyrophosphate (Cocarboxylase) :
FUJIFILM Wako Pure Chemical Corporation#031–038334–AA: NACALAI TESQUE, INC. Special grade #01907–52
POD: Sigma Chemical Co. Type Ⅱ #P–8250
DMA: FUJIFILM Wako Pure Chemical CorporationSpecial grade #044–02763FAD (2Na) : Kyowa Hakko Co., Ltd.
Potassium pyruvate:FUJIFILM Wako Pure Chemical CorporationEDTA (2Na・2H2O) : KISHIDA CHEMICAL Co., Ltd. #060–29133
for biochemistry #166–08351
Enzyme solution
-
Accurately weigh about 20 mg of the sample and add enzyme dilution buffer to make a total of 20 ml. Dilute it with enzyme solution buffer to adjust the concentration as required.
Procedure
- Mix reaction mixture Ⅰ , substrate solution and reaction mixture Ⅱ in a ratio of 6 : 1 : 3. Pipette accurately 1.0 ml of the mixture into a small test tube and preincubate at 37℃.
- After 5 min, add exactly 20 μl of enzyme solution and mix to start the reaction at 37℃.
※ In the case of a test blank, add 20 μl of enzyme dilution buffer in place of enzyme solution. - At 10 min after starting the reaction, add 2.0 ml of the reaction stopper to stop the reaction.
- After 5 min, measure the absorbance at 565 nm.
△A = (As−Ab) ≦ 0.400 AbsAbsorbance sample : As blank : Ab
Calculation
Activity (U/mg of powder) = {(△A/10) /(23.56×1/2)}× 3.02/0.02 × 1/x| 23.56 : | millimolar extinction coefficient of quinoneimine dye at 565 nm
( cm2 /μmole) |
| 1/2 : | a multiplier derived from the fact that 2 mole of |
-
H2O2 produces 1 mole of quinoneimine dye 10 : reaction time (min) 3.02 : final volume (ml) 0.02 : volume of enzyme solution (ml) X : concentration of the sample in enzyme solution ( mg/ml)
Storage
-
Storage at −20℃ in the presence of a desiccant is recommended. Enzyme activity will be retained for at least one year under this condition (Figure 5) .
References
- Harger, L. P., Geller, D. M. and Lipmann, F. (1954) Fed.
Proc., 13, 734–738. - Lipmann, F. (1940) J. Biol. Chem., 134, 463–464.
- Harger, L. P. and Lipmann, F. (1955) Methods Enzymol.,
Vol. 1, 482. - Sedewitz, B., Schleifer, K. H. and Gotz, F. (1984) J.
Bacteriol., 160, 273–278. - Sedewitz, B., Schleifer, K. H. and Gotz, F. (1984) J.
Bacteriol., 160, 462–465.
POPG 活性測定法 (Japanese)
試薬液
- 反応試薬混合液Ⅰ
1M KH2PO4 – NaOH 緩衝液pH6.7 0.20 ml 精製水 0.22 ml 15mM 4–AA 溶液 0.10 ml 100U/ml POD 溶液 1) 0.05 ml 10mM チアミンピロリン酸溶液 0.02 ml 1mM FAD 溶液 0.01 ml 1) : 100U/ml POD 溶液
POD 1,000 単位 (PPU) を精製水10ml で溶解する。 - 反応試薬混合液Ⅱ
0.2% (V/V) DMA 溶液 0.20 ml 0.1M 塩化マグネシウム溶液 0.10 ml - 基質溶液
1M ピルビン酸カリウム溶液 - 反応停止液
0 . 1M E D T A を含むマックイルバイン緩衝液
pH5.50~5.55 - 酵素溶解希釈用液
10 μ M FAD を含む10mM KH2 PO4 – NaOH 緩衝液pH7.0 - 試薬
チアミンピロリン酸 (コカルボキシラーゼ) :富士フイルム和光純薬製 #031–03833POD: シグマ製 Type Ⅱ #P–8250
4–AA: ナカライテスク製 特級 #01907–52
DMA (N,N’– ジメチルアニリン) :富士フイルム和光純薬製 特級 #044–02763FAD (フラビンアデニンジヌクレオチド・2Na) :協和発酵製
- ピルビン酸カリウム:
富士フイルム和光純薬製 生化学用 #166–08351
EDTA (エチレンジアミン四酢酸・2Na・2H2O) :キシダ化学製 #060–29133
酵素試料液
- 検品約20mg を精密に量り、酵素溶解希釈用液で溶解して全容20ml とする。
その液を酵素溶解希釈用液で適宜希釈する。
測定操作法
- 反応試薬混合液Ⅰと基質溶液及び反応試薬混合液Ⅱを6:1:3 に混合し、その混合液1.0ml ずつを正確に小試験管へ分注して37℃で予備加温する。
- 5 分経過後、酵素試料液20 μl を加えて混和し、37℃で反応を開始する。
※ 盲検は酵素試料液の代わりに酵素溶解希釈用液20μl を加える。 - 10 分経過後、反応停止液2.0ml を加えて混和し、反応を停止する。
- 5 分後、565nm における吸光度を測定する。
求められた吸光度を試料液はAs、盲検液はAb とする。
ΔA= (As−Ab) ≦ 0.400 Abs
計算
活性 (U/mg) = {(ΔA/10)/ (23.56 × 1/2)}× 3.02/0.02 × 1/x| 23.56 : | キノンイミン色素の565nm におけるミリモル分子吸光係数 (cm2 / μmole) |
| 1/2 : | H2O2 2 モルからキノンイミン色素1 モルが生成することによる係数 |
| 10 : | 反応時間 (min) |
| 3.02 : | 反応総液量 (ml) |
| 0.02 : | 反応に供した酵素試料液量 (ml) |
| X : | 酵素試料液中の検品濃度 (mg/ml) |