ASCORBATE OXIDASE [ASOM]
from Acremonium sp.
(L–Ascorbate: oxygen oxidoreductase, EC 1.10.3.3)
L– Ascorbic Acid + 1/2 O2 Dehydroascorbic Acid + H2O
Preparation and Specification
- Appearance
- : Light blue amorphous powder, lyophilized
- Specific activity
- : More than 200 U/mg solid
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 80 kDa (gel filtration)
- Isoelectric point
- : pH 4.0
- Michaelis constants
- : Ascorbic acid (pH 7.0) 1.0 × 10-4 M
Ascorbic acid (pH 4.0) 3.8 × 10-4 M
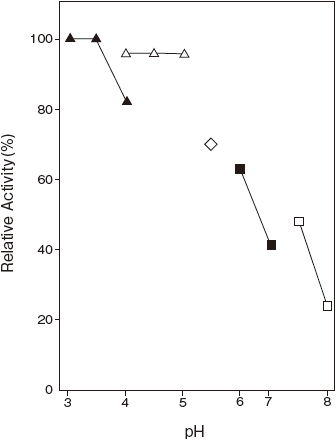
- Optimum pH
- : 4.0–4.5 (Acetate buffer) Figure 1
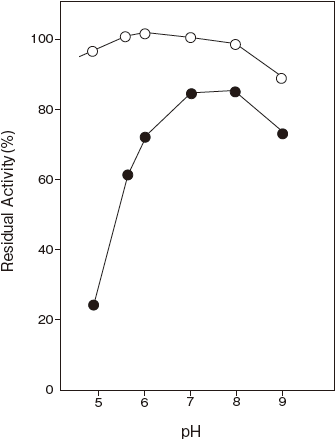
- pH stability
- : 6.0–9.0 (30℃, 24 hr) Figure 2
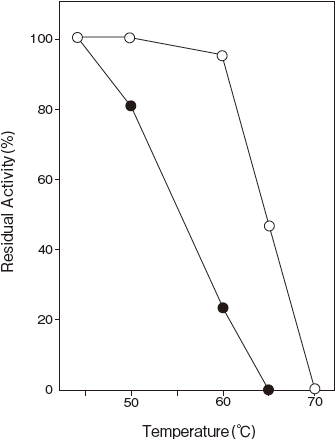
- Thermal stability
- : Stable at 50℃ and below (pH 7.0, 10 min) Figure 3
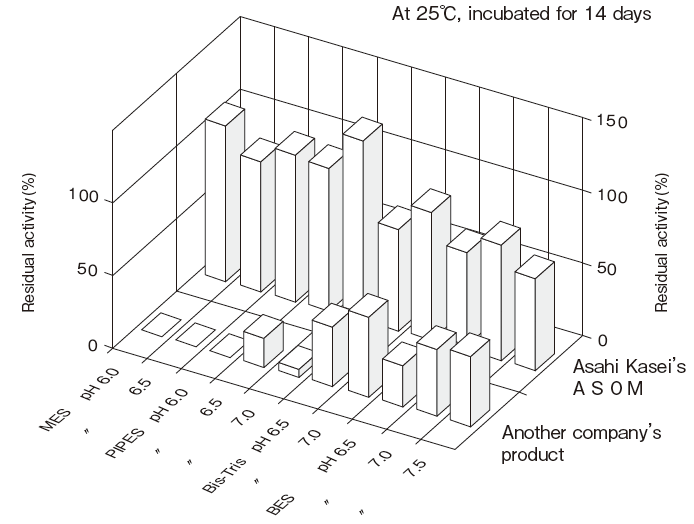
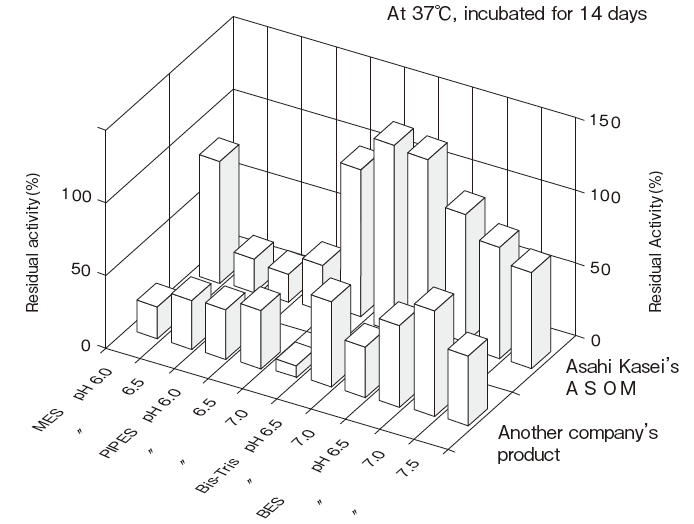
- Liquid stability
- : See Figure 4
- Effect of metal ions
- : See Table 2
- Stabilizers
- : BSA, Mannitol
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 80 kDa (gel filtration)
- Isoelectric point
- : pH 4.0
- Michaelis constants
- : Ascorbic acid (pH 7.0) 1.0 × 10-4 M
Ascorbic acid (pH 4.0) 3.8 × 10-4 M
- Optimum pH
- : 4.0–4.5 (Acetate buffer) Figure 1
- pH stability
- : 6.0–9.0 (30℃, 24 hr) Figure 2
- Thermal stability
- : Stable at 50℃ and below (pH 7.0, 10 min) Figure 3
- Liquid stability
- : See Figure 4
- Effect of metal ions
- : See Table 2
- Stabilizers
- : BSA, Mannitol
Applications for Diagnostic Test
This enzyme is useful for avoidance from interference of ascorbic acid on diagnostic assay such as blood, uric acid, TG, TC and creatinine.
Table 1. Substrate specificity
| Substrate | Relative activity (%) |
|---|---|
| L-Ascorbate | 100 |
| I-Naphthol | 0 |
| Hydroquinone | 0 |
| Catechol | 0 |
| Pyrogallol | 0 |
Table 2. Effect of metal ions on ASOM activity
| Metal ion | Concentration | Relative activity (%) |
|---|---|---|
| None | - | 100 |
| KCl | 10 mM | 100 |
| NaCl | 10 mM | 100 |
| EDTA | 1mM | 100 |
Assay
Principle
-
The assay is based on the decrease in absorbance at 245 nm as ascorbic acid is oxidized in the following reaction:
| ASOM | ||
| Ascorbic acid+1/2 O2 | → | Dehydroascorbic acid+H2O |
Unit definition
-
One unit is defined as the amount of enzyme which oxidizes 1 μmole of ascorbate to dehydroascorbate per minute at 30 ℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture
Dilute substrate solution for stock 1) with dilution buffer 2)
to make a 20–fold solution.1) : Substrate solution for stock (10 mM L–ascorbic acid solution)
Dissolve 176 mg of L–ascorbic acid and 37 mg of EDTA with 100 ml of 1mM HCl.
EDTA: Ethylenediamine tetraacetic acid2) : Dilution buffer
90mM KH2PO4–5mM Na2HPO4 buffer containing 0.45 mM EDTA
- Reaction stopper
0.2 N HCl solution - Enzyme dilution buffer
10 mM Na2HPO4 solution containing 0.05% (W/V) BSA - Reagents
L–Ascorbic acid:FUJIFILM Wako Pure Chemical CorporationEDTA (2Na・2H2O) : KISHIDA CHEMICAL Co., Ltd.
Special grade # 012–04802#060–29133BSA: Millipore Fraction V pH 5.2 #81–053
Enzyme solution
-
Accurately weigh about 20 mg of the sample and add enzyme dilution buffer to make a total of 20 ml.
Dilute it with enzyme dilution buffer to adjust the concentration to within 0.08–0.35 U/ml.
Procedure
- Pipette accurately 1.0 ml of reaction mixture into a
small test tube and preincubate at 30℃. - After 5 min, add exactly 100 μl of enzyme solution and
mix to start the reaction at 30℃. - At 5 min after starting the reaction, add 3.0 ml of the reaction stopper to stop the reaction.
※ In the case of a test blank, add 100 μl of enzyme dilution buffer after adding reaction stopper in place of enzyme solution.
-
Measure the absorbance at 245 nm.
0.100Abs ≦ ΔA = Ab−As ≦ 0.420AbsAbsorbance sample : As blank : Ab
Calculation
-
Activity (U/mg of powder) = {(△ A/5)/10.0} × 4.10/0.10 × 1/x
10.0 : millimolar extinction coefficient of ascorbic acid at 245 nm at pH 1.0 (cm2/μmole) 5 : reaction time (min) 4.10 : final volume (ml) 0.10 : volume of enzyme solution (ml) X : concentration of the sample in enzyme solution (mg/ml)
Storage
-
Storage at -20℃ in the presence of a desiccant is recommended.
References
- Murao, S., et al (. 1991) Agric. Biol. Chem., 55 (6) , 1693–1694.
- Nakamura, T., Makino, N. and Ogura, Y. (1968) J.
Biochem., 64, 189. - Aikazyan, V. Ts. and Nalbandyan, R. M. (1979)
FEBS Lett, 104, 127. - White, G. A. and Smith, F. G. (1961) Nature, 190, 187.
ASOM 活性測定法 (Japanese)
試薬液
- 保存基質溶液 (10mM L–アスコルビン酸)
L–アスコルビン酸 176mg とEDTA 37mg を1mM
HCl 100ml で溶解する。 - 反応試薬混合液
上記の保存基質溶液を希釈用液※) で20 倍に希釈する。※) : 希釈用液
0.45mM EDTA を含む90mM KH2PO4–5mM
Na2HPO4 溶液 - 反応停止液
0.2N HCl 液 - 酵素溶解希釈用液
0.05%(W/V)BSAを含む10mM Na2HPO4溶液 - 試薬
L–アスコルビン酸:富士フイルム和光純薬製 特級 #012–04802EDTA (エチレンジアミン四酢酸・2Na・2H2O) :キシダ化学製 #060–29133BSA: Millipore 製 Fraction V pH5.2 #81–053
酵素試料液
- 検品約20mg を精密に量り、酵素溶解希釈用液に溶解して全容20ml とする。
- その液を酵素溶解希釈用液で0.08~0.35U/ml 濃度となるように適宜希釈する。
測定操作法
- 小試験管に反応試薬混合液1.0ml を正確に分注して30℃で予備加温する。
- 5 分経過後、酵素試料液100 μl を加えて混和し、30℃で反応を開始する。
-
5 分経過後、反応停止液3.0ml を加えて混和し、反応を停止する。
※ 盲検は反応停止後に酵素試料液100 μl を加える。 - 245nm における吸光度を測定する。
求められた吸光度を試料液はAs、盲検液はAb とする。
0.100Abs ≦ ΔA = Ab−As ≦ 0.420Abs
計算
-
以下の計算式に従い、活性 (U/mg) を計算する。
活性 (U/mg) = {(△ A/5)/10.0} × 4.10/0.10 × 1/x10.0 : pH 1 の条件でアスコルビン酸の245nm におけるミリモル分子吸光係数 ( cm2/ μmole) 5 : 反応時間 (min) 4.10 : 反応総液量 (ml) 0.10 : 反応に供した酵素試料液量 (ml) X : 酵素試料液中の検品濃度 (mg/ml)