Preparation and Specification
- Appearance
- : White amorphous powder, lyophilized
- Specific activity
- : More than 30 U/mg solid
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 41 kDa(gel filtration)
- Isoelectric point
- : pH 4.8±0.2
- Michaelis constants
- : Androsterone 2.1 × l0-4M
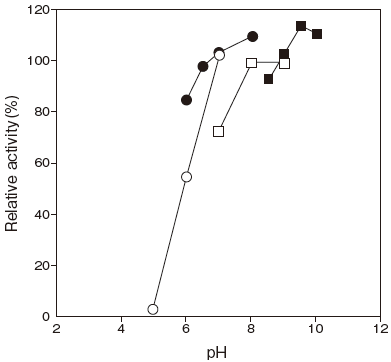
- Optimum pH
- : 8.0–10.0Figure 1
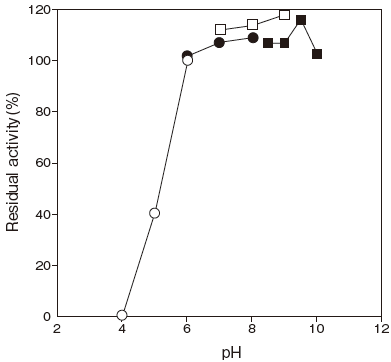
- pH stability
- : 6.0–10.0(37℃, 10 min)Figure 2
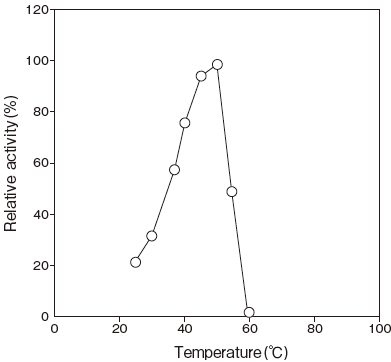
- Optimum temperature
- : 50℃(Phosphate buffer) Figure 3
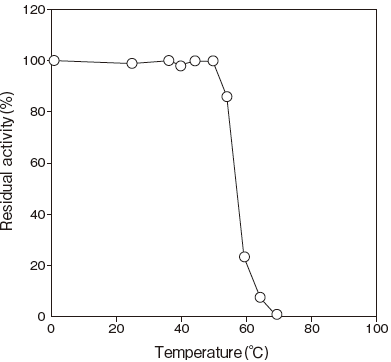
- Thermal stability
- : Stable at 50℃ and below(pH 8.0, 10 min)Figure 4
- Effect of metal ions
- : See Table 2
- Effect of detergents
- : See Table 3
- Inhibitor
- : MnCl2
Applications for Diagnostic Test
This enzyme is useful for enzymatic cycling determination of bile acid when coupled with thio–NAD and NADH.
Table 1. Substrate specificity
| Substrate (0.95mM) |
Relative activity (%) |
|---|---|
| Cholic acid | 100 |
| Androsterone | 131 |
| Deoxycholic acid | 115 |
| Chenodeoxycholic acid | 89.0 |
| Glycocholic acid | 103 |
| Taurocholic acid | 99.0 |
| Taurodeoxycholic acid | 128 |
Table 2. Effect of metal ions on 3α–HSDⅡ activity
| Metal ion | Relative activity (%) |
|---|---|
| None |
100 |
| NaCl | 105 |
| KCl | 102 |
| LiCl | 101 |
| MgCl2 | 106 |
| MnCl2 | 16.0 |
| CaCl2 | 105 |
Table 3. Effect of detergents on 3α–HSDⅡ activity
| Detergent | Relative activity (%) |
|---|---|
| None | 100 |
| Triton X–100 | 71.0 |
| Triton X–305 | 71.0 |
| Triton X–114 | 71.0 |
| Adekanol SO–120 | 104 |
| Adekanol NP–720 | 75.0 |
| Adekanol B–795 | 78.0 |
| Emulgen B–66 | 75.0 |
| Emulgen 911 | 76.0 |
| Emulgen 709 | 84.0 |
| Emulgen 810 | 50.0 |
| Emulgen 109P | 112 |
| Rheodol 460 | 71.0 |
| Rheodol TWL–103 | 72.0 |
Assay
Principle
-
The assay is based on the increase in absorbance at 550 nm as formazan dye is formed in the following reactions:
| 3α–HSD Ⅱ | ||
| Cholic acid+NAD+ | → | 3–Oxocholic acid+NADH+H+ |
| DI | ||
| NADH+H++NTB | → | NAD++NTBH2 |
NAD : Nicotineamido adenine dinucleotide,
NTB : Nitrotetrazolium blue
DI : Diaphorase
Unit definition
One unit is defined as the amount of enzyme which oxidizes 1 μmole of cholic acid to 3–oxocholic acid per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture
10 mM NAD solution 0.05 ml 0.25%(W/V)NTB solution 0.05 ml 100 U/ml DI solution 0.025 ml 2%(W/V)Triton X–100 solution 0.10 ml 0.2 M Tris–HCl buffer pH 8.0 0.10 ml Distilled water 0.175 ml 1): 100 U/ml DI solution
Dissolve 100 U of DI with 1 ml of 10 mM Tris–HCl buffer pH 8.0. - Substrate solution(20 mM Androsterone)
Dissolve 23 mg of androsterone with 4 ml of methanol. - Reaction stopper
0.5%(W/V)Sodiumdodecyl sulfate(SDS)solution - Enzyme dilution buffer
10 mM Tris–HCl buffer pH 8.0 - Reagents
NAD: NACALAI TESQUE, INC. #24334–84
- NTB: Dojindo Laboratories #344–02033
DI: Asahi Kasei Pharma Corporation #T–06
Triton X–100: The Dow Chemical Company
Androsterone: Sigma Chemical Co. #A–9755
SDS(Sodium Dodecyl Sulfate):
NACALAI TESQUE, INC. Extra pure #31606–75
Enzyme solution
-
Accurately weigh about 20 mg of the sample and add enzyme dilution buffer to make a total of 20 ml. Dilute it with enzyme dilution buffer to adjust the concentration as required.
Procedure
- Pipette accurately 0.5 ml of reaction mixture into a
small test tube, then add 20 μl of enzyme solution into the same test tube and preincubate at 37℃.※ In the case of a test blank, add 20 μl of enzyme dilution buffer in place of enzyme solution. - After 5 min, add exactly 25 μl of substrate solution and mix to start the reaction at 37℃.
- At 5 min after starting the reaction, add 2.5 ml of the reaction stopper to stop the reaction.
- Measure the absorbance at 550 nm.
△A =(As−Ab)≦ 0.20 AbsAbsorbance sample : As blank : Ab
Calculation
- Activity(U/mg of powder)= {(△A/5)/(16.7)} × 3.045/0.02 × 1/x
-
16.7 : millimolar extinction coefficient of NTBH2 at 550 nm(cm2/ μmole) 5 : reaction time(min) 3.045 : final volume(ml) 0.02 : volume of enzyme solution(ml) X : concentration of the sample in enzyme solution ( mg/ml)
Storage
-
Storage at −20℃ in the presence of a desiccant is recommended.
References
- Suzuki, K. and Tamaoki, B.(1974)J. Steroid Biochem., 5,
249–256. - Inano, H., Hayashi, S. and Tamaoki, B.(1977)J. Steroid Biochem., 8, 41–46.
- Shikita, M. and Talalay, P.(1979)Anal. Biochem., 92, 286–292.
- Uwajima, T., Takayama, K. and Terada, O.(1978)Agric.
Biol. Chem., 42, 1577–1583. - Talalay, P.(1962)Methods Enzymol., 5, 512.
- Mowszowicz, I. and Bardin, C. W.(1974)Steroids, 23, 793–807.
- Nimrod, A., Lamprecht, S. A. and Lindner, R. H.(1975)J. Steroid Biochem., 6, 1205–1209.
- Nozu, K., Inano, H. and Tamaoki, B.(1974)Proteins Nucleic Acids and Enzymes(Japan), 19, 397–410.
3α–HSD Ⅱ活性測定法(Japanese)
試薬液
- 反応試薬混合液
10mM NAD 溶液 0.05 ml 0.25%(W/V)NTB 溶液 0.05 ml 100U/ml DI 溶液1) 0.025 ml 2%(W/V)トリトンX–100 溶液 0.10 ml 0.2M トリス−HCl 緩衝液pH8.0 0.10 ml 精製水 0.175 ml 1): 100U/ml DI 溶液
DI 100 単位(U)を10mM トリス−HCl 緩衝液 pH8.0 1ml で溶解する。 - 基質溶液(20mM アンドロステロン溶液)
アンドロステロン23mg をMeOH4ml で溶解する。 - 反応停止液
0.5%(W/V)SDS 溶液 - 酵素溶解希釈用液
10mM トリス−HCl 緩衝液pH8.0 - 試薬
NAD(ニコチンアミドアデニンジヌクレオチド):ナカライテスク製 #24334–84NTB(ニトロテトラゾリウムブルー):同仁化学製 #344–02033DI(ジアフォラーゼ):旭化成ファーマ製 #T–06
トリトンX–100:Dow Chemical 製
アンドロステロン:シグマ製 #A–9755
SDS(ドデシル硫酸ナトリウム):ナカライテスク製 一級 #31606–75
酵素試料液
- 検品約20mg を精密に量り、酵素溶解希釈用液で溶解して全容20ml とする。
その液を酵素溶解希釈用液で適宜希釈する。
測定操作法
- 小試験管に反応試薬混合液0.5ml を正確に分注し、後に酵素試料液20 μl を正確に分注して37℃で予備加温する。
※ 盲検は酵素試料液の代わりに酵素溶解希釈用液20μl を加える。
- 5 分経過後、基質溶液25 μl を正確に加えて混和し、37℃で反応を開始する。
- 5 分経過後、反応停止液2.50ml を正確に加えて混和し、反応を停止する。
- 550nm における吸光度を測定する。
求められた吸光度を試料液はAs、盲検液はAb とする。
ΔA =( As−Ab) ≦ 0.20 Abs
計算
活性(U/mg)= {(ΔA/5)/(16.7)} × 3.045/0.02 × 1/x| 16.7 : | NTBH2 の550nm におけるミリモル分子吸光係数 (cm2 / μmole) |
| 5 : | 反応時間(min) |
| 3.045 : | 反応総液量(ml) |
| 0.02 : | 反応に供した酵素試料液量(ml) |
| X : | 酵素試料液中の検品濃度(mg/ml) |