LIPASE [LPBP]
from Microorganism
(Triacylglycerol acylhydrolase, EC 3.1.1.3)
(Triacylglycerol lipase)
Triglyceride + 3H2O → Glycerol + 3 Fatty Acid
| ★ | Advantages | |
| ① | Low adsorption onto cuvette | |
| ② | Stability in solution | |
Preparation and Specification
- Appearance
- : White to light brownish amorphous powder, lyophilized
- Specific activity
- : More than 800 U/mg solid
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 55 kDa (SDS–PAGE)
- Isoelectric point
- : pH 4.9±0.2
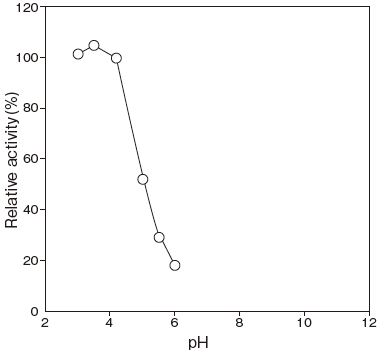
- Optimum pH
- : 4.2Figure 1
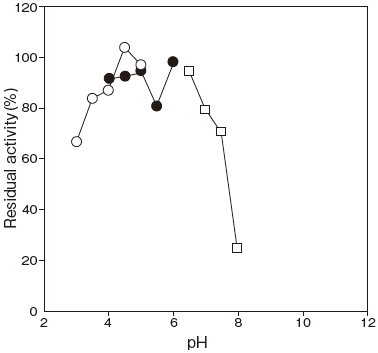
- pH stability
- : pH 3.5–7.0 (45℃, 60 min) Figure 2
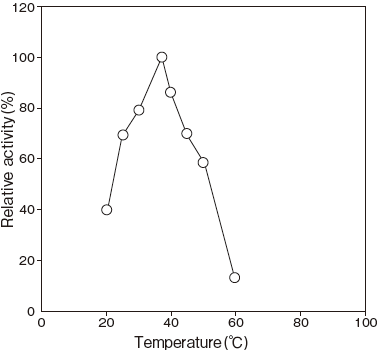
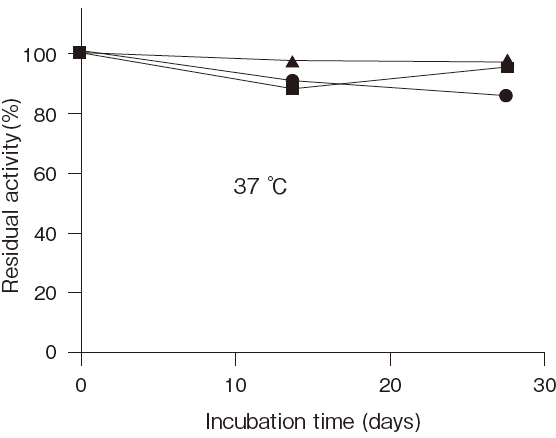
- Optimum temperature
- : 37℃ (Phosphate buffer) Figure3
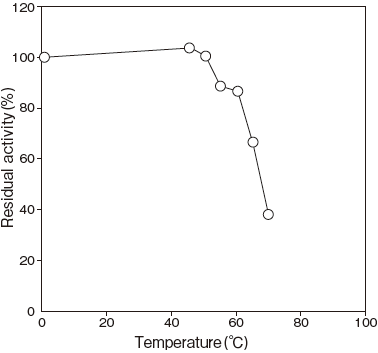
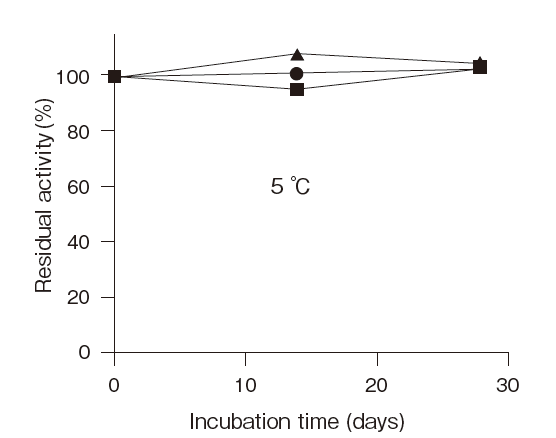
- Thermal stability
- : Stable at 37℃ and below (pH 7.5, 30 min) Figure4
- Effect of metal ions
- : See Table 2
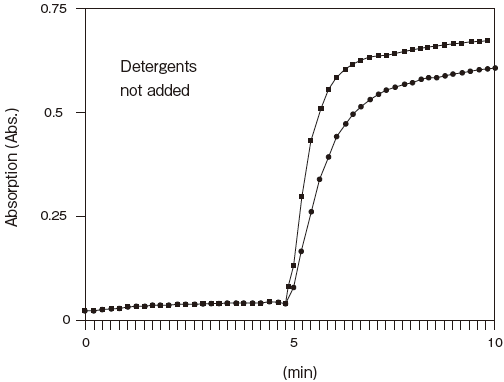
- Low adsorption
- : See Figure 5
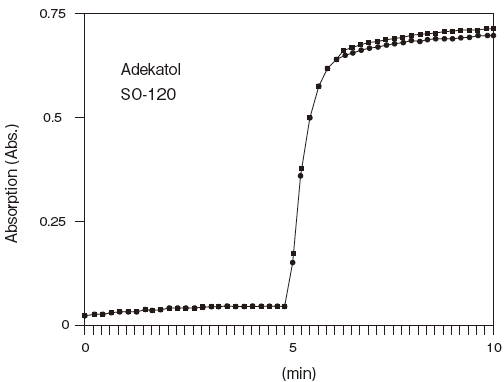
- Liquid stability
- : See Figure 6
- High reactivity after
long storage - : See Figure 7
- Effect of various
chemicals - : See Table 3
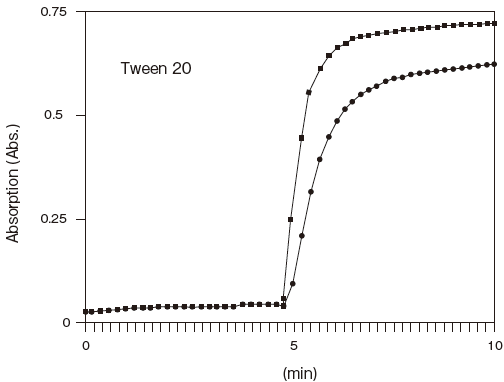
- Effect of detergents
- : See Table 4
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of triglyceride.
| LPBP | ||
| TG + 3H2O | → | Glycerol + 3 Fatty acid |
| GKZ | ||
| Glycerol + ATP | → | G - 3 - P + ADP |
| GPOSP | ||
| G -3 - P + O2 | → | DHAP + H2O2 |
| POD | ||
| 2H2O2 + 4-AA + Phenol | → | Quinoneimine dye + 4 H2O |
TG : Triglyceride
DHAP : Dihydroxyacetone phosphate
Table 1. Substrate specificity
| Substrate | Relative activity (%) |
|---|---|
| Triolein | 100 |
| Trimyristin | 14.8 |
| Trilaurin | 34.9 |
| Tricaprylin | 92.2 |
| Tricaprin | 87.1 |
| Tricaproin | 13.5 |
| Tributyrin | 38.0 |
| Triacetin | 0 |
Table 3. Effect of detergents on LPBP activity
| Substrate | Relative activity (%) |
|---|---|
| None | 100 |
| Adekanol NP695 | 91.9 |
| Adekanol NP720 | 96.2 |
| Adekanol SO120 | 86.7 |
| Adekanol B795 | 88.1 |
| Triton X305 | 91.9 |
| Emulgen B66 | 87.9 |
Table 2. Efect of various chemicals on LPBP activity
| Additives | Concentration | Relative activity (%) |
|---|---|---|
| None |
- |
100 |
| NiCl | 1mM | 94 |
| MnCl2 | 1mM | 90 |
| (NH4)2 SO4 | 1mM | 92 |
| MgCl2 | 1mM | 98 |
| ZnCl | 1mM | 100 |
| ZnSO4 | 1mM | 100 |
| Ba(CH3COO)2 | 1mM | 101 |
| CaCl2 | 1mM | 97 |
| MoSO4 | 1mM | 100 |
| CuSO4 | 0.5mM | 103 |
| CuCl2 |
0.5mM |
99 |
| FeCl3 |
1mM |
97 |
| CoCl2 |
1mM |
106 |
| Li2CO3 |
1mM |
105 |
| EDTA |
1mM |
102 |
| KCl |
100mM |
103 |
| NaCl |
100mM |
100 |
| NaN3 |
0.05% |
98 |
| NaF | 20mM | 4 |
Assay
Principle
- The assay is based on the titration of fatty acids liberated in the following reactions:
| LPBP | ||
| Triglyceride+3 H2O | → | Glycerol+3 Fatty acid |
| ( Titration) | ||
| Fatty acid+NaOH | → | Na–Fatty acid+H2O |
Unit definition
-
One unit is defined as the amount of enzyme which liberates 1 μmole of fatty acid per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- McIlvaine buffer pH 4.2
Mix 0.2 M Na2HPO4 and 0.1 M citrate solution and adjust pH to 4.2 at 25℃. - Substrate suspension (Olive oil and Adekatol SO–120)
50g of Olive oil (Japanese Pharmacopoeia grade and 50g of Adekatol SO–120 are suspended with 150 ml of distilled water. - Reaction stopper
Mixture of ethanol and acetone (1:1) - Indicator
1% (W/V) Phenolphthalein–ethanol solution - Titration solution
50 mM NaOH solution - Enzyme dilution buffer
0.1 M KH2PO4–NaOH buffer, pH 6.0 containing
0.1% (W/V) BSA and 0.1% (W/V) NaN3 - Reagents
Olive oil: (Japanese Pharmacopoeia grade)
Ethanol: (Japanese Pharmacopoeia grade)
Adekatol SO–120: ADEKA CORPORATION
BSA: Millipore Fraction V pH5.2 #81–053
Enzyme solution
- Accurately weigh about 10 mg of the sample and add enzyme dilution buffer to make a total 10 ml. Dilute it with enzyme dilution buffer to adjust the concentration as required.
Procedure
- Pipette accurately 5 ml of substrate suspension and 2 ml of McIlvaine buffer into test tube (24 mm i.d. × 200 mmH) and mix to preincubate at 37℃.
- After 10 min, add 0.50 ml of enzyme solution and mix to start the reaction.
※ In the case of a test blank, add 0.50 ml of enzyme dilution buffer in place of enzyme solution. - After 20 min, stop the reaction with 16 ml of reaction stopper.
- Add 3 drops of indicator and titrate the whole mixture under nitrogen gas bubbling.
※ End point of titration: Appearance of the same color as that of the blank
△V = (Vs-Vc) ≦ 1.5 mlTitration volume :Vs blank : Vc
Calculation
-
Activity (U/mg of powder) = {(△V×F)/20}× 50× 1/0.5 × 1/x
20 : : reaction time (min) F : factor of titration solution (50 mM NaOH) 50 : concentration (mM) of titration solution
( 50 mM NaOH)0.5 : the volume of enzyme solution (ml) X : concentration of the sample in enzyme solution ( mg/ml)
Storage
- Storage at -20℃ in the presence of a desiccant is recommended. Enzyme activity will be retained for at least one year under this condition.
References
- Yamaguchi, T., Muroya, N., Isobe, M. and Sugiura,
M. (1973) Agric. Biol. Chem., 37, 999–1005. - Sugiura, M., Isobe, M., Muroya, N. and Yamaguchi, T. (1974) Agric. Biol. Chem., 38, 947–952.
- Sugiura, M. and Isobe, M. (1974) Biochem. Biophys.
Acta, 341, 195–200.
- Sugiura, M. and Isobe, M. (1975) Chem. Pharm. Bull., 23, 1226–1230.
- Horiuchi, Y., Koga, H. and Gocho, S. (1976) J. Biochem., 80, 367–370.
- Saiki, T., Takagi, Y. Suzuki, T., Narasaki, T., Tamura, G. and Arima, K. (1969) Agric. Biol. Chem., 33, 414.
LPBP 活性測定法 (Japanese)
試薬液
- McIlvaine 緩衝液 pH4.2
0.2M Na2HPO4 溶液と0.1M クエン酸溶液を混合してpH4.2 (25℃) に調整する。 - 基質懸濁液 (オリーブ油とアデカトールSO–120 の懸濁液)
「局方」オリーブ油50.0g とアデカトールSO–120
50.0g を精製水150ml に懸濁する。 - 反応停止液
エタノール-アセトン (1:1) 混液 - 指示液
1% (W/V) フェノールフタレン-エタノール溶液 - 滴定液
50mM NaOH 液 - 酵素溶解希釈用液
0.1% (W/V) BSA と0.1% (W/V) NaN3 を含む
0.1M KH2PO4–NaOH 緩衝液 pH6.0 - 試薬
オリーブ油:「局方」
エタノール:「局方」
アデカトールSO–120:ADEKA 製
BSA: Millipore 製 Fraction V pH5.2 #81–053
酵素試料液
- 検品約10mg を精密に量り、酵素溶解希釈用液で溶解して全容10ml とする。
その液を酵素溶解希釈用液で適宜希釈する。
測定操作法
- 試験管 (24mm i.d × 200mm H) に基質懸濁液5.0mlとMcIlvaine 緩衝液2.0ml を正確に分注して攪拌混和後、37℃で予備加温する。
- 10 分経過後、酵素試料液0.50ml を加えて混和し、37℃で反応を開始する。
※ 盲検は酵素試料液の代わりに酵素溶解希釈用液0.50ml を加える。 - 20 分経過後、反応停止液16.0ml を加えて反応を停止する。
- 指示液3 滴を加えてN2 ガスで攪拌しながら滴定液で滴定する。
ΔV = ( Vs−Vc) ≦ 1.5 ml※ 滴定の終点は盲検時と同色 (淡赤色) を呈した時点とする。
求められた滴定量を試料液はVs、盲検液はVc とする。
計算
活性 (U/mg) = {(ΔV×F)/20}×50 × 1/0.5 × 1/x| 20 : | 反応時間 (min) |
| F : | 滴定液 (50mM NaOH) のFactor |
| 50 : | 滴定液 (50mM NaOH) の濃度 (mM) |
| 0.5 : | 反応に供した酵素試料液量 (ml) |
| X : | 酵素試料液中の検品濃度 (mg/ml) |
Copyright © Asahi Kasei Pharma Corporation. All rights reserved.