CHOLESTEROL OXIDASE [CONⅡ]
from Rhodococcus sp.
(Cholesterol: oxygen oxidoreductase, EC 1.1.3.6)
Cholesterol +O2 → △4–Cholesten–3–one + H2O2
Preparation and Specification
- Appearance
- : Yellowish solution
- Specific activity
- : More than 500 U/ml
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 61.8 KDa (SDS–PAGE)
- Isoelectric point
- : pH 4.5
- Michaelis constants
- : Cholesterol 6.0 × 10-5M
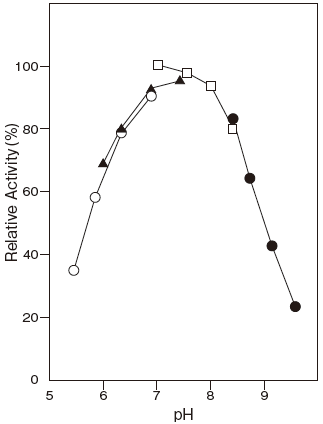
- Optimum pH
- : 7.0–7.5Figure 1
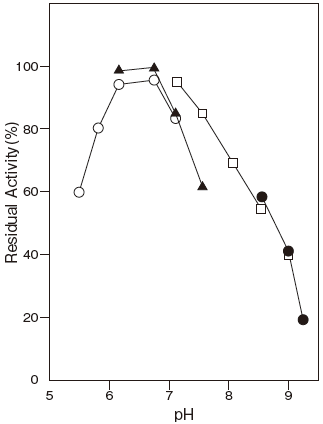
- pH stability
- : 5.7–7.8 (65℃, 10 min) Figure 2
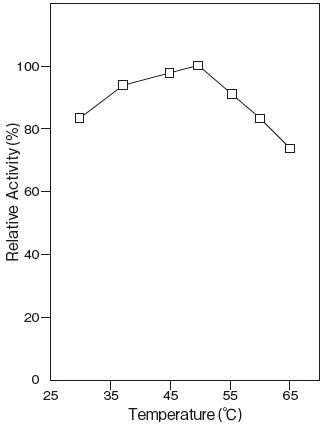
- Optimum temperature
- : 50℃ (Tris–HCl buffer) Figure 3
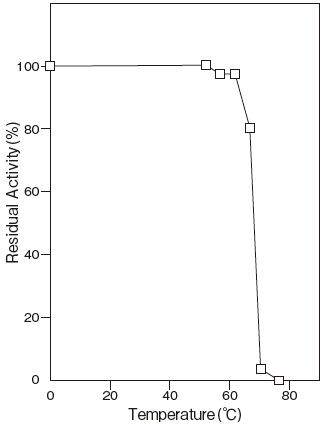
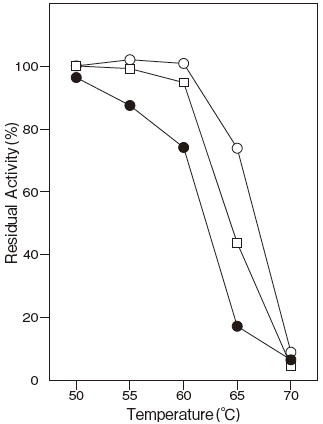
- Thermal stability
- : Stable at 65℃ and below (pH 7.0, 10 min) Figure 4 and Figure 5
- Effect of detergents
- : See Table 2
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of total cholesterol,
| CEN | ||
| Cholesterol ester + H2O | → | Cholesterol + FFA |
| CON Ⅱ | ||
| Cholesterol + O2 | → | Cholestenone + H2O2 |
| POD | ||
| 2 H2O2 + 4-AA + Phenol | → | Quinoneimine dye + 4 H2O |
FFA: Free fatty acid
Table 1. Substrate specificity
| Substrate (1mM) | Relative activity (%) |
|---|---|
| Cholesterol | 100 |
| β–Cholesterol | 93 |
| Pregnenolone | 98 |
| Dehydro–iso–androsterone | 10 |
| β–Sitosterol | 94 |
| Stigmasterol | 66 |
| Androsterol | 2 |
| Teststerone | 1 |
| Cholic acid | 3 |
Table 2. Effect of detergents on CON II activity
| Detergents (0.1%) | Relative activity (%) |
|---|---|
| Triton X–100 | 100 |
| Emulgen 810 | 101 |
| Emulgen 911 | 113 |
| Emulgen 709 | 107 |
| Emulgen 109P | 118 |
| Adekatol SO–120 | 100 |
| RHEODOL 460 | 63 |
| SM 1080 | 122 |
Assay
Principle
-
The assay is based on the increase in absorbance at 240 nm as △4–cholesten–3–one is produced in the following reaction:
| CON Ⅱ | ||
| Cholesterol+O2 | → | △4–Cholesten–3–one+H2O2 |
Unit definition
-
One unit is defined as the amount of enzyme which liberates 1 μmole of △4–cholesten–3–one per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Substrate solution (6 mM cholesterol solution)
Dissolve 232 mg of cholesterol with isopropanol to make a total of 100 ml.
- Enzyme dilution buffer
0.1 M KH2 PO4–Na2 HPO4 buffer pH 7.0 containing
0.05% (W/V) Triton X–100 - Reagents
Cholesterol : NACALAI TESQUE, INC. Special grade
#08721Triton X–100 : The Dow Chemical Company
Enzyme solution
-
Dilute accurately 0.5 ml of the sample with enzyme dilution buffer to make a 40–fold solution. Dilute it with enzyme dilution buffer to adjust the concentration to within 0.1–0.2 U/ml.
Procedure
- Pipette accurately 3.0ml of enzyme dilution buffer and 50 μl of enzyme solution and preincubate at 37℃.
※ In the case of a test blank, add 50 μl of enzyme dilution buffer in place of enzyme sdution. - After 5 min, add 50 μl of substrate solution and mix to start the reaction at 37℃.
- After starting the reaction, measure the rate of increase per minute in absorbance at 240nm. The rate must be measured within the linear portion of the absorbance curve.
0.010 Abs/min ≦ △ A/min = (As/min-Ab/min)Absorbance sample : As/min blank : Ab/min
≦ 0.060 Abs/min
Calculation
-
Activity (U/ml) = {(△ A/min)/12.2} × 3.10/0.05 × D
12.2 : millimolar extinction coefficient of △4–Cholesten–
3–one at 240 nm( cm2 /μmole)3.10 : final volume (ml) 0.05 : volume of enzyme solution (ml) D : times of dilution in enzyme solution
Storage
-
Storage at -80℃ in the presence of a desiccant is recommended. Enzyme activity will be retained for at least one year under this condition.
References
- Richmond, W. (1973) Clin. Chem., 19, 1350.
- Flegg, H. M. (1973) Ann. Clin. Biochem., 10, 79.
- Alain, C. C. et. al. (1973) Clin. Chem., 20, 470.
- Tarbutton, P. N. and Gunter, C. R. (1974) Clin. Chem., 20, 724.
- Nomoto, S. (1976) Rinsho Kensa, 20, 688.
- Kameno, K., Nakano, N. and Baba, S. (1976) Jap. J. Clin. Path., 24, 650.
CON Ⅱ活性測定法 (Japanese)
試薬液
- 基質溶液 (6mM コレステロール溶液)
コレステロール232mg をイソプロパノールに溶解して全容100ml とする。 - 酵素溶解希釈用液
0.05% (W/V) トリトンX–100 を含む
0.1M KH2PO4–Na2HPO4 緩衝液 pH7.0 - 試薬
コレステロール:ナカライテスク製 特級#08721トリトンX–100:Dow Chemical 製
酵素試料液
- 検品0.5ml を酵素溶解希釈用液で40 倍に稀釈する。
その液を酵素溶解希釈用液で約0.1–0.2U/ml 濃度となるように適宜希釈する。
測定操作法
- 小試験管に酵素溶解希釈用液3.0ml と酵素試料液50μl を正確に加え37℃で予備加温する。
※ 盲検は酵素試料液の代りに酵素溶解希釈用液50 μlを加える。
- 5 分経過後、基質溶液50 μl を正確に加えて混和し、37℃で反応を開始する。
- 反応開始後、240nm における吸光度を測定して直線的に反応している1 分間当たりの吸光変化を求める。
求められた吸光度変化を試料液はAs/min,盲検液は
Ab/min とする。
0.010 Abs/min ≦ △ A/min = (As/min-Ab/min)
≦ 0.060 Abs/min
計算
活性 (U/ml) = {(△ A/min)/12.2} × 3.10/0.05 × D| 12.2 : | Δ4– コレステン–3– オンの240nm におけるミリモル分子吸光係数 (cm2 / μmole) |
| 3.10 : | 反応総液量 (ml) |
| 0.05 : | 反応に供した酵素試料液量 (ml) |
| D : | 酵素試料液の稀釈倍率 |