GLYCEROL KINASE [GKZ]
from Flavobacterium meningosepticum
(ATP: Glycerol–3–phosphotransferase, EC 2.7.1.30)
Glycerol + ATP → L–α–Glycerolphosphate + ADP
| ★ | Advantage | |
| ① | Thermal stability | |
| ② | Stability in solution | |
| ③ | Antiseptic stability | |
Preparation and Specification
- Appearance
- : White to light grayish amorphous powder, lyophilized
- Specific activity
- : More than 70 U/mg solid
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 150 kDa (TSK G3000SWXL)
50 kDa (SDS–PAGE)
- Isoelectric point
- : pH 4.3
- Michaelis constants
- : Glycerol 8.8 × 10-5M
ATP 3.0 × 10-5M
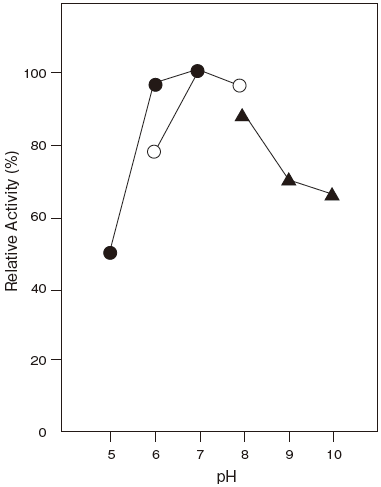
- Optimum pH
- : 8.0Figure 1
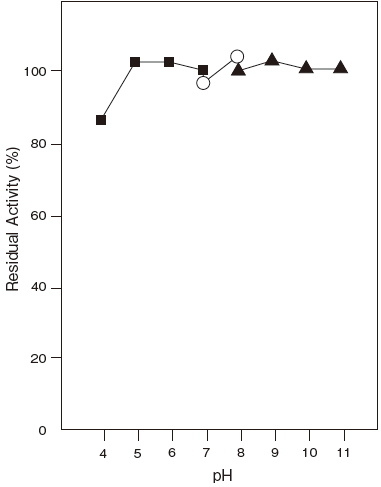
- pH stability
- : 5.0–11.0 (37℃, 60 min) Figure 2
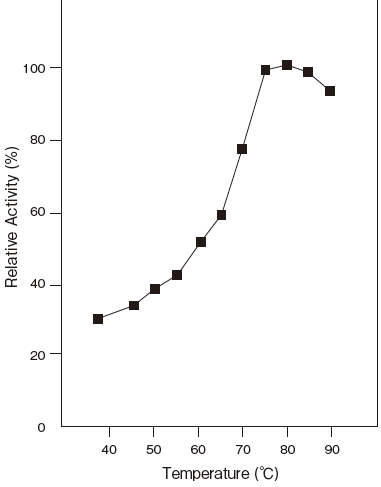
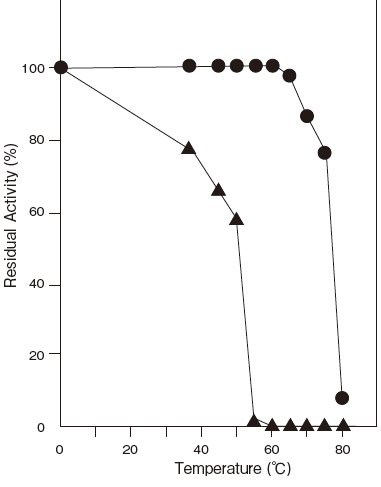
- Optimum temperature
- : 80℃ (Tris–HCl buffer) Figure3
- Thermal stability
- : Stable at 60℃ and below (pH 6.5, 10 min)
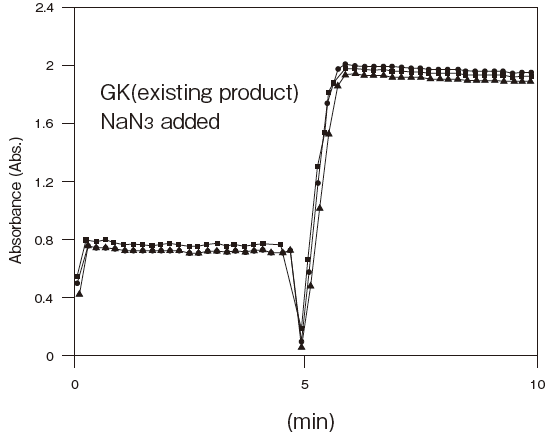
Comparison data between GKZ and GKFigure4
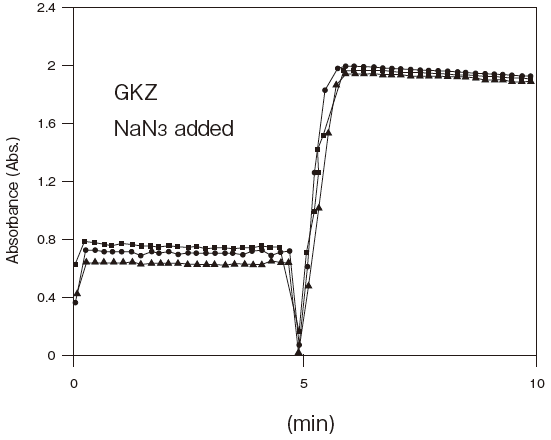
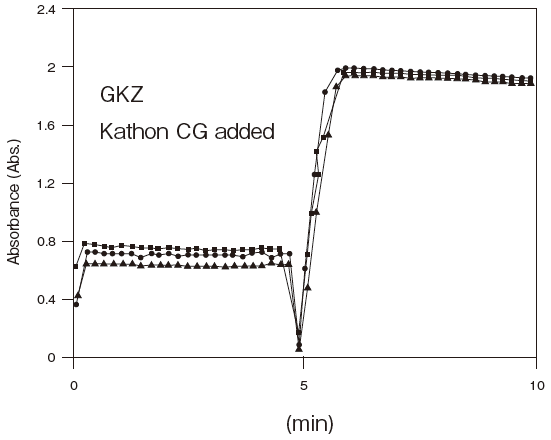
- Antiseptic stability
- : See Figure 5
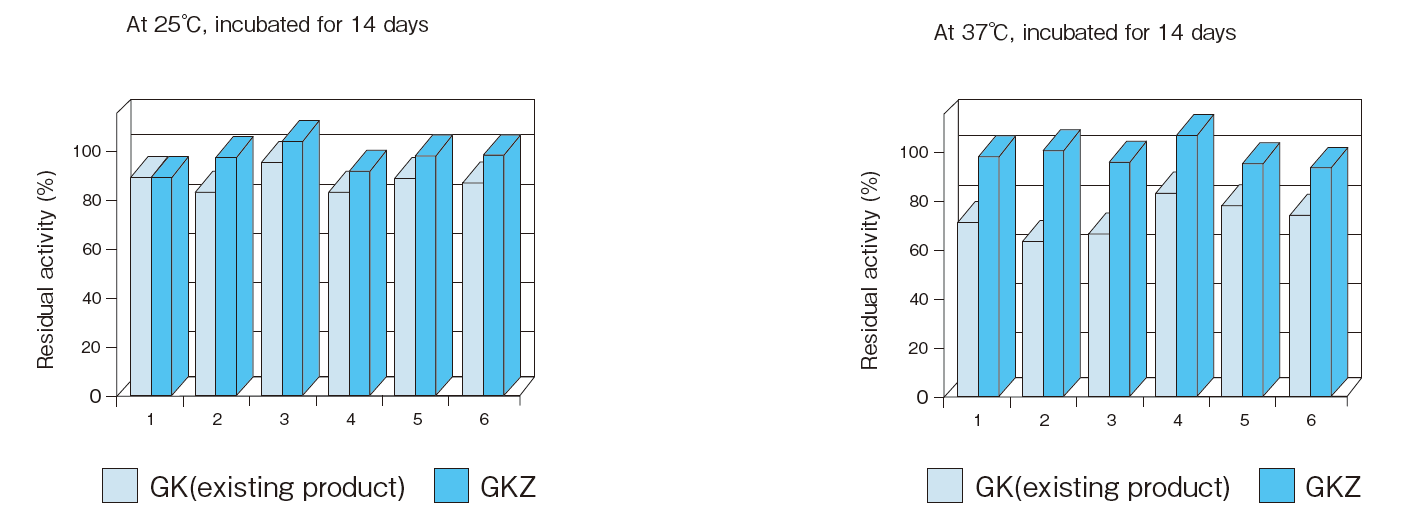
- Reactivity after
long incubation - : See Figure 6
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of triglyceride.
| LPBP | ||
| TG + 3 H2O | → | Glycerol + 3 Fatty acid |
| GKZ | ||
| Glycerol + ATP | → | G - 3 - P + ADP |
| GPOSP | ||
| G - 3 - P + O2 | → | DHAP + H2O2 |
| POD | ||
| H2O2 + 4-AA + Phenol | → | Quinoneimine dye + 4 H2O |
TG: Triglyceride, DHAP:Dihydroxyacetone phosphate
Table 1. Substrate Specificity
| Substrate | Relative activity (%) |
|---|---|
| Glycerol |
100 |
| Glycerol–α–monochlorohydrin |
0 |
| Ethylene glycol |
0 |
| 1,3–Propanediol |
0 |
| 1,3–Butanediol |
0 |
| 1,4–Butanediol |
0 |
| 1,2–Butanediol |
0 |
| d–Mannitol |
0 |
| d–Sorbitol |
0 |
| d–Glucose |
0 |
| Robitol | 0 |
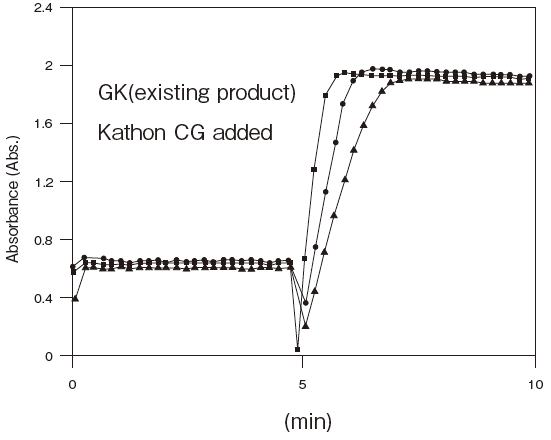
Fig.5 Resistance of GKZ against antiseptics
2:Benzalkonium chloride (0.001%)
3:Gentamicin (0.0005%)
5:Sulphamethizole (0.0005%)
6:Dihydrostreptomycin (0.0005%)
|
Storage conditions: | 25℃, 1.2 U/ml GKZ or 0.4 U/ml GK(existing product) |
|||||||
| 50 mM PIPES pH 6.5, 3 mM ATP, 2 mM MgCl2, 0.05% antliseptic |
|||||||||
| Autoanalyzer: HITACHI 7150 |
|||||||||
| Sample: Glycerol solution | |||||||||
| Notes: | The reason for the high absorbance number comes from co-existence of TOOS and 4-amino antipyrine in the first reagents. |
Assay
Principle
-
The assay is based on the increase in absorbance at 366 nm as NADH is produced in the following reactions:
| GKZ | ||
| Glycerol+ATP | → | Glycerol–3–P+ADP |
| G3PDH | ||
| Glycerol–3–P+NAD+ | → | Dihydroxyacetone phosphate+NADH+H+ |
ATP : Adenosine triphosphate
NAD : Nicotineamido adenine dinucleotide
G3PDH : Glycerol–3–phosphate dehydrogenase
Unit definition
-
One unit is defined as the amount of enzyme which converts 1 μmole of glycerol to glycerol–3–phosphate per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Substrate–coenzyme mixture
80 mM ATP solution pH 7.0
-
0.1 M NAD solution 2.0 ml 0.1 M Glycerol solution 2.0 ml - Buffer solution
Glycine–hydrazine buffer containing 1.5mM MgCl 2 pH 9.8
Dissolve 12.5g hydrazine hydrate and 1.5g glycine with about 80ml distilled water. Add 1.5ml 0.1M MgCl2 solution and adjust pH to 9.8 with 1N HCl at 25℃. Add distilled water to make a total of 100ml.
- G3PDH solution
Use G3PDH (NH4) 2SO4 suspension (undiluted) - Enzyme dilution buffer
10 mM KH2PO4–NaOH buffer pH 7.0 containing
10 mM glycerol - Reagents
ATP: Kyowa Hakko Co., Ltd. Hydrazine hydrate:Tokyo Kasei Kogyo Co., Ltd. Purity 79% #H0204NAD: NACALAI TESQUE, INC. #24334–84
G3PDH: Roche Diagnostics GmbH(NH4) 2SO4 suspension #10127752001
Enzyme solution
-
Accurately weigh about 20 mg of the sample and add enzyme dilution buffer to make a total of 20 ml. Dilute it with enzyme dilution buffer to adjust the concentration as required.
Procedure
- Pipette accurately 1.80 ml of buffer into a small test tube, add 0.15 ml of substrate/coenzyme mixture and 20 μl of G3PDH, mix immediately.
After mixing, preincubate at 37℃. - After 5 min, add 30 μl of enzyme solution and mix to start the reaction at 37℃.
※ In the case of a test blank, add 30 μl of enzyme dilution buffer in place of enzyme solution. - After starting the reaction, measure the rate of increase per minute in absorbance at 366 nm. The rate must be measured within the linear portion of the absorbance curve.
△A/min = (As/min−Ab/min) ≦ 0.050 Abs/minAbsorbance sample : As/min blank : Ab/min
Calculation
-
Activity (U/mg of powder) = {(△A/min)/3.3} × 2.00/0.03 × 1/x
3.3 : milimolar extinction coefficient of NADH at 366 nm (cm2/μmole) 2.00 : final volume (ml) 0.03 : volume of enzyme solution (ml) X : concentration of the sample in enzyme solution ( mg/ml)
Storage
-
Storage at −20℃ in the presence of a desiccant is recommended.
References
-
Sakasegawa, S., Yoshioka, I., Koga, S., Takahashi, M., Matsumoto, K., Misaki, H. and Ohshima, T. (1998) Biosci, Biotechnol, Biochem., 62, 2388-2395
GKZ 活性測定法 (Japanese)
試薬液
- 基質、補酵素混合液
80mM ATP 溶液 pH7.0
2.0 ml 0.1M NAD 溶液 2.0 ml 0.1M グリセロール溶液 2.0 ml - 緩衝液 (1.5mM MgCl 2 を含むグリシン–ヒドラジン緩衝液 pH9.8)
ヒドラジン一水和物 (純度79%) 12.5g とグリシン1.5g および0.1M 塩化マグネシウム溶液 1) 1.5ml を精製水80ml に溶解した後、1N HCl でpH9.8 (25℃) に調整し、精製水で全容100ml とする。 1) : 塩化マグネシウム溶液
塩化マグネシウム203mg を精製水で溶解して全容10ml とする。
又はヒドラジン一水和物 (純度79%) 12.5g とグリシン1.50g 及び0.1M 塩化マグネシウム溶液1) 1.50ml を精製水で溶解して全容100ml とし、pH が9.8 ± 0.05 (25℃) であればそのまま使用する。 - G3PDH 溶液
ロシュ製のG3PDH 懸濁液をそのまま使用する。 - 酵素溶解希釈用液
10mM グリセロールを含む10mM KH2PO4–NaOH
緩衝液 pH7.0 - 試薬
ATP (アデノシン三リン酸・2Na・3H2O) :協和発酵製ヒドラジン一水和物:東京化成工業製 純度79% #H0204NAD (ニコチンアミドアデニンジヌクレオチド) :ナカライテスク製 #24334–84
- G3PDH (グリセロ–3–リン酸脱水素酵素) :
ロシュ製 硫安懸濁液
酵素試料液
- 検品約20mg を精密に量り、酵素溶解希釈用液で溶解して全容20ml とする。
その液を酵素溶解希釈用液で適宜希釈する。
測定操作法
- 小試験管に緩衝液1.80ml を正確に分注して、加温する直前に基質、補酵素混合液0.15ml とG3PDH 溶液20 μl を分注して混和した後、直ちに37℃で予備加温する。
- 5 分経過後、酵素試料液30 μl を正確に加えて混和し、直ちに37℃で反応を開始する。
※ 盲検は酵素試料液の代わりに酵素溶解希釈用液30μl を加える。 - 反応開始後、366nm における吸光度を測定して直線的に反応している1 分間当たりの吸光度変化を求める。
求められた吸光度変化を試料液はAs/min、盲検液は
Ab/min とする。
ΔA/min = (As/min−Ab/min) ≦ 0.050 Abs/min
計算
活性 (U/mg) = {(△ A/min)/3.3} × 2.00/0.03 × 1/x| 3.3 : | NADH の366nm におけるミリモル分子吸光係数 (cm2 / μmole) |
| 2.00 : | 反応総液量 (ml) |
| 0.03 : | 反応に供した酵素試料液量 (ml) |
| X : | 酵素試料液中の検品濃度 (mg/ml) |