
Close
FAQ
Close
Ever since the early 1970s when we succeeded in industrializing enzymes for lipid analysis, we have supplied more than one hundred enzymes for diagnostic products.
We have also been working closely together with our customers from the initial stages of development, and have introduced new measurement methods to the world. We also offer many products that are compliant with REACH.
We have our uniquely owned microorganism library and genome database that we can scan through to enable us to swiftly develop and propose new and unique enzymes that meet your needs. We also have a uniquely developed host microbial group, which allows us to manufacture even difficult-to-express enzymes at a practical level.
We draw out the enzyme production capability of the host microorganism to its maximum level by making use of culture development techniques that have been optimized for each host microbial group. In addition, our technological capabilities in building flexible purification processes tailored to the characteristics of the enzymes enable their rapid mass production.
We make use of our many years of experience in enzyme production and have a strict quality management structure to achieve stable production of high-quality enzymes.
Disruption of SMC-related genes promotes recombinant cholesterol esterase production in Burkholderia stabilis.
Applied Microbiology and Biotechnology vol. 106,24 (2022): 8093-8110.
Konishi, Kenji et al. DOI: 10.1007/s00253-022-12277-3
Novel enzymatic method for assaying Lp-PLA2 in serum.
Clinica Chimica Acta; International Journal of Clinical Chemistry vol. 481 (2018): 184-188.
Yamaura, Saki et al. DOI: 10.1016/j.cca.2018.03.012
Characterization and application of a novel nicotinamide mononucleotide adenylyltransferase from Thermus thermophilus HB8.
Journal of Bioscience and Bioengineering vol. 125,4 (2018): 385-389.
Konishi, Kenji et al. DOI: 10.1016/j.jbiosc.2017.10.017

Diagnostic products manufacturers

Biosensor manufacturers

Manufacturers of raw materials for pharmaceutical additives
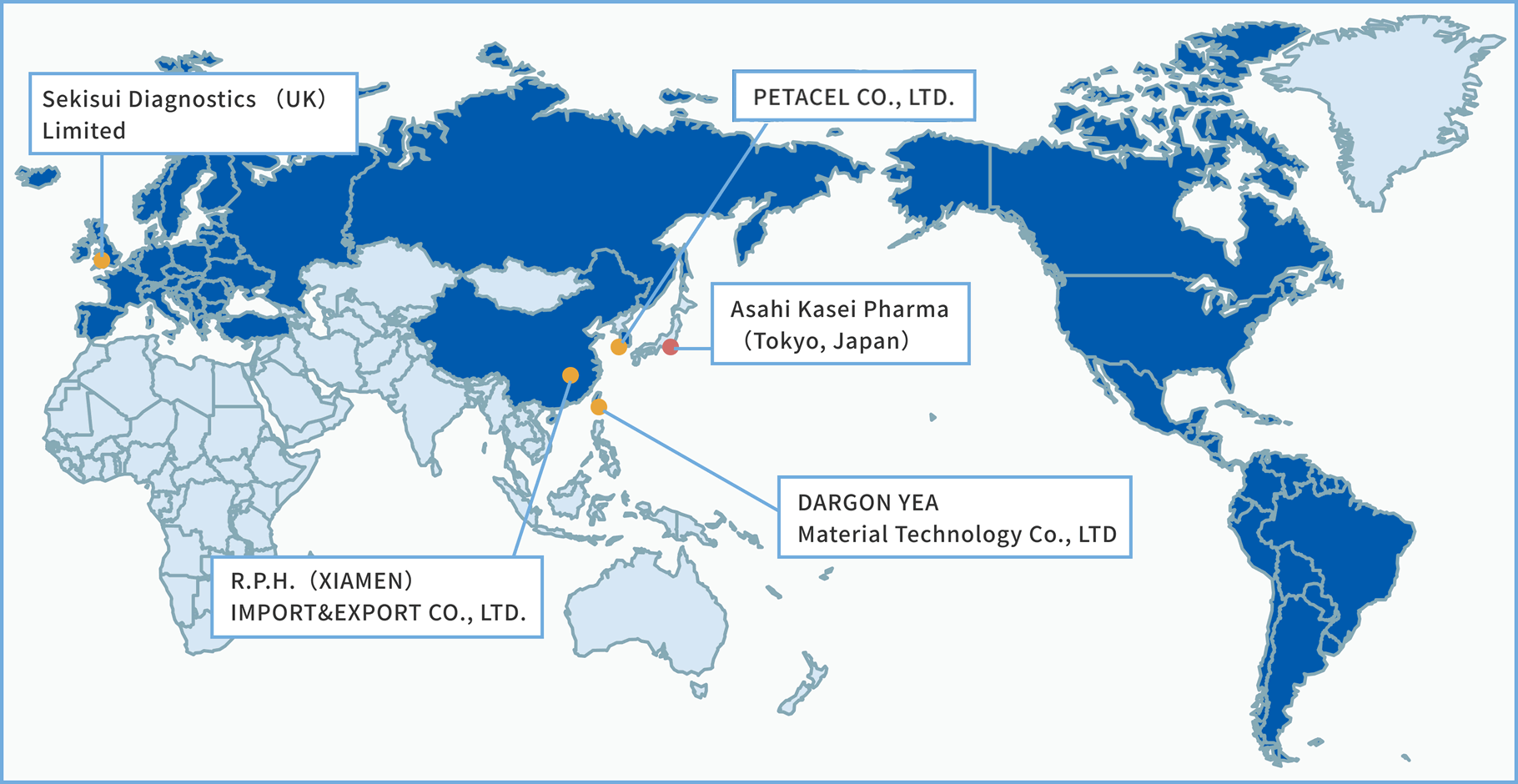
Note: If you are in Japan or in areas not listed below, please contact us via the inquiry form.
URL:http://www.yutaikang.com
Sales areas:China
URL:https://www.longyeind.com/
Sales areas:Taiwan
URL:https://petacel.com
Sales areas:South Korea
URL:https://sekisuidiagnostics.com/
Sales areas:Albania/
URL:https://sekisuidiagnostics.com/
Sales areas:Argentina/