GLUCOSE DEHYDROGENASE [FAD-GDHⅡ]
from Microorganism
(FAD dependent glucose dehydrogenase, EC 1.1.5.9)
D‒Glucose + acceptor → D‒glucono-1,5-lactone + reduced acceptor
Preparation and Specification
- Appearance
- : Light yellow to yellow lyophilizate
- Specific activity
- : More than 400 U/mg solid
Properties
- Molecular weight
- : 66 kDa (SDS-PAGE, as deglycosylated form)
- Michaelis constant
- : 1.3 × 10-2M (glucose)
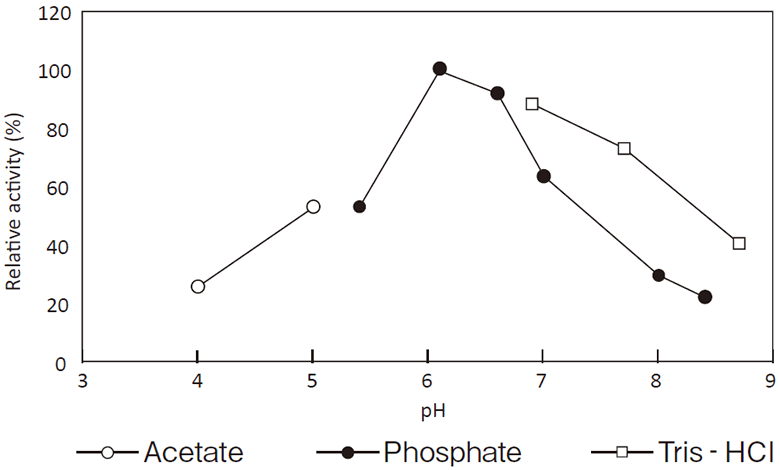
- Optimum pH
- : See Figure 1
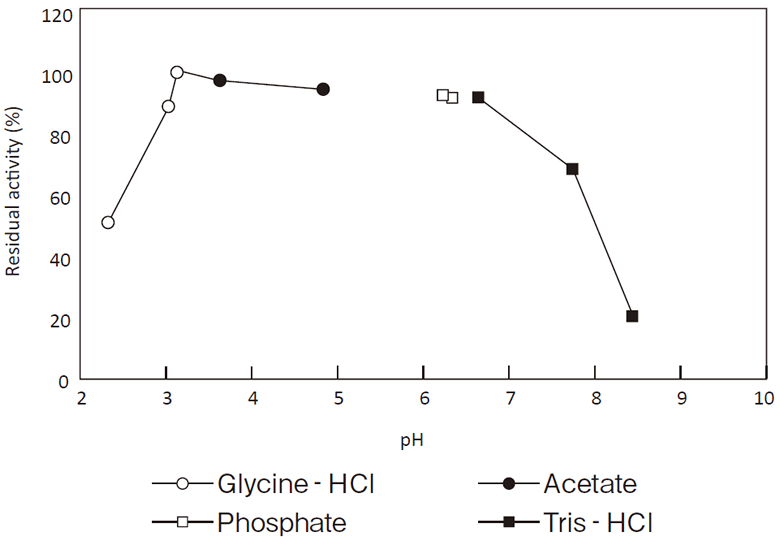
- pH stability
- : See Figure 2
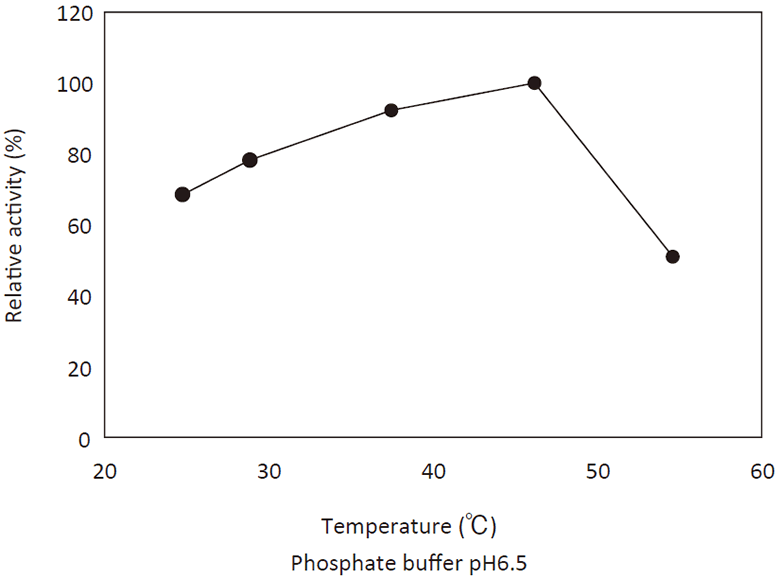
- Optimum temperature
- : See Figure 3
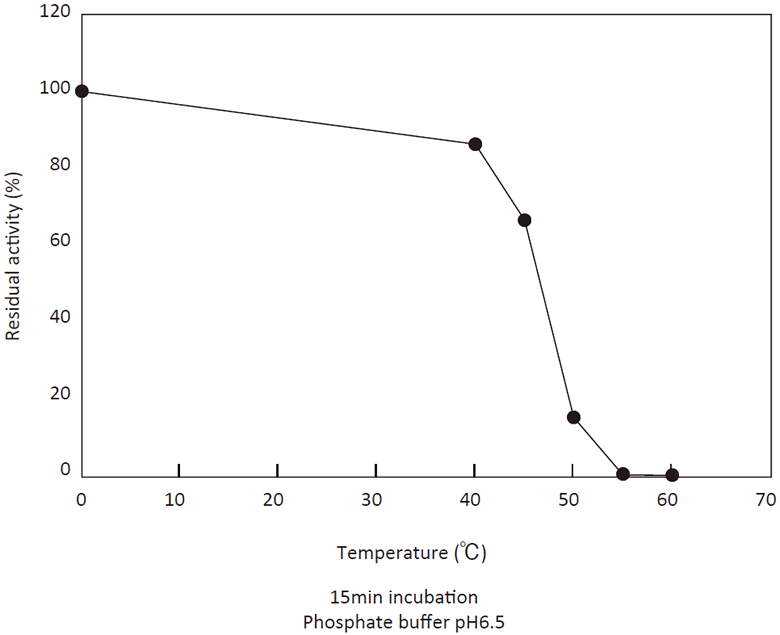
- Thermal stability
- : See Figure 4
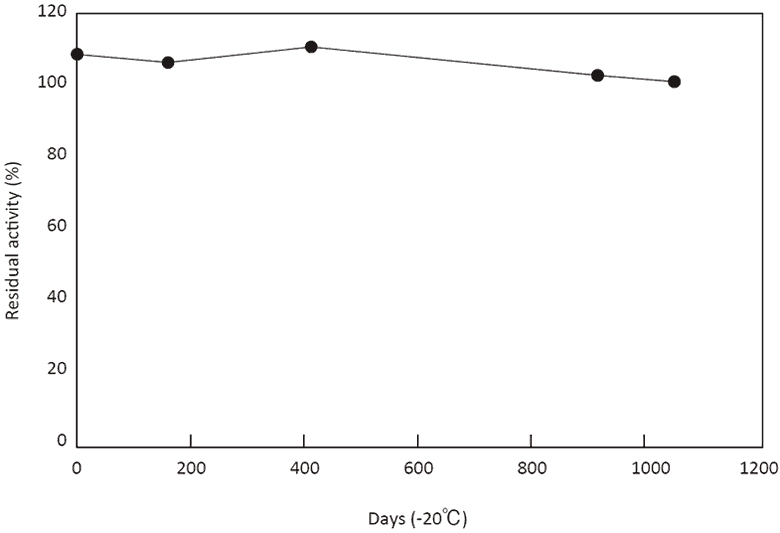
- Stability (solid form)
- : See Figure 5
- Substrate specificity
- : See Table 1
- Light stability
- : See Table 2
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of glucose.
Table 1. Substrate specificity
| Substrate | FAD-GDHⅡ | FAD-GDH from Aspergillus sp. |
|---|---|---|
| Glucose | 100 | 100 |
| Galactose | 0.6 | 0.3 |
| Xylose | 2.4 | 13.5 |
| Maltose | 0.4 | 0 |
| Mannose | 1.0 | 1.3 |
| 2-deoxy-glucose | 31.8 | 29.2 |
Table 2. Light stability
| Form of FAD-GDH | Light source | Residual activity (%) |
|---|---|---|
| Powder | LED | 93.2 |
| Fluorescent lamp | 93.7 | |
| Solution (1mg/ml, water) |
LED | 95.7 |
| Fluorescent lamp | 96.4 |
Radiation for 24 hours
Assay
Principle
-
The assay is based on the decrease in absorbance at
600 nm as the reduction of 2,6-dichloroindophenol in the
following reactions:
| FAD-GDH Ⅱ | ||
| D-Glucose + PMS | → | D-Glucono-1,5-lactone + PMS (red) |
| PMS (red) + DCIP | → | PMS + DCIP (red) |
PMS : Phenazine methosulfate
DCIP : 2,6-Dichloroindophenol
Unit definition
-
One unit is defined as the amount of enzyme which
converts 1 μmole of D-Glucose to D-Glucono-1,5-lactone
per minute at 37 ℃ under the conditions specified in the
assay procedure.
Reagents
- Reaction mixture
0.1 M NaH2PO4‒Na2HPO4 buffer pH 6.5 containing 0.222 M D-Glucose and 0.0833% Triton X-100 - 20 mM PMS solution
0.2 N HCl solution - 2.0 mM DCIP solution
- Enzyme dilution buffer
50 mM NaH2PO4‒Na2HPO4 buffer pH 6.5 containing 0.1 % BSA and 0.1 % Triton X-100 - Reagents
Triton X-100 : The Dow Chemical Company
BSA : Millipore Fraction V pH5.2 #81053
D-Glucose : FUJIFILM Wako Pure Chemical Corporation #049-31165
PMS (Phenazine methosulfate) : Tokyo Chemical Industry #P0083
DCIP (2,6-Dichloroindophenol) : Sigma-Aldrich #119814
Enzyme solution
-
Accurately weigh about 10 mg of the sample and add enzyme dilution buffer to make a total of 20 ml. Dilute it with enzyme dilution buffer to adjust the concentration as required.
Procedure
- Pipette accurately 1.8 ml of reaction mixture and 20 μl of enzyme solution into a small test tube. Add 100 μl of 20 mM PMS solution, mix and preincubate at 37 ℃.
※ In the case of a blank test, add 20 μl of enzyme dilution buffer at this time. - After 3 min, add 100 μl of 2.0 mM DCIP solution and mix to start the reaction at 37 ℃.
- After starting the reaction, measure the rate of increase per minute in absorbance at 600 nm. The rate must be measured within the linear portion of the absorbance curve.
△ A/min =(As/min−Ab/min)≦ 0.1Abs/minAbsorbance sample : As/min blank : Ab/min
Calculation
-
Activity(U/mg of powder)= {(△A/min)/(16.3)} × 2.02/0.02 × 1/x
16.3 : millimolar extinction coefficient of DCIP at 600 nm (cm2/μmole) 2.02 : final volume (ml) 0.02 : volume of enzyme solution (ml) X : concentration of the sample in enzyme solution (mg/ml)
Storage
-
Storage at -20 ℃ in the presence of a desiccant is recommended.
References
- Matsushita, K. et al. (1980) Agric. Biol. Chem. 44, 1505-1512.
- Shinagawa, E. et al. (1990) J. Biochem. 107, 863-867.
FAD-GDH Ⅱ活性測定法(Japanese)
試薬液
- 反応試薬混合液
0.222 M グルコース及び0.0833%トリトンX-100含む0.1 M リン酸緩衝液 pH 6.5 - 20 mM PMS 溶液
- 2.0 mM DCIP 溶液
- 酵素溶解希釈用液
0.1 % BSA 及び0.1 % トリトンX-100 を含む50mM リン酸緩衝液 pH 6.5 - 試薬
トリトンX-100:Dow Chemical製
BSA (Bovine serum albumin) Fra. V, pH5.2:Millipore製 #81053
グルコース:富士フイルム和光純薬製 特級 #049-31165
PMS (フェナジンメチルスルファート):東京化成製 #P0083
DCIP (2,6-ジクロロインドフェノールナトリウム塩):シグマ製 #119814
酵素試料液
- 検品約10 mg を精密に量り、酵素溶解希釈用液に溶解して全容20 ml とする。その液を酵素溶解希釈用液で適宜希釈する。
測定操作法
- 小試験管に反応試薬混合液1.8 ml 及び酵素試料液20μl を正確に分注し、20 mM PMS 溶液100 μl を加えて混和し、37 ℃で予備加温する。
※ 盲検は酵素試料液の代わりに酵素溶解希釈用液20μl を加える。 - 3 分経過後、2.0 mM DCIP 溶液100 μl を加えて混和し、37 ℃で反応を開始する。
- 反応開始後、600 nm における吸光度を測定して直線的に反応している1 分間当たりの吸光度変化を求める。
求められた吸光度変化を試料液についてはAs/min、盲検液についてはAb/min とする。
ΔA/min =(As/min − Ab/min)≦ 0.1 Abs/min
計算
活性(U/mg)= {(ΔA/min)/(16.3)} × 2.02/0.02 × 1/x| 16.3 : | DCIP の600nm におけるミリモル分子吸光係数(cm2/μmole) |
| 2.02 : | 反応総液量(ml) |
| 0.02 : | 反応に供した酵素試料液量(ml) |
| X : | 酵素試料液中の検品濃度(mg/ml) |