ACYL–CoA OXIDASE [ACOD]
from Arthrobacter sp.
(Acyl–CoA: oxygen 2–oxidoreductase, EC 1.3.3.6)
Acyl–CoA + O2 trans– 2,3–dehydroacyl–CoA + H2O2
Preparation and Specification
- Appearance
- : Yellowish amorphous powder, lyophilized
- Specific activity
- : More than 20 U/mg solid
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 210 kDa (Sephadex G–150)
- Isoelectric point
- : pH 4.7
- Michaelis constants
- : Palmitoyl–CoA 2.0 × 10-5M
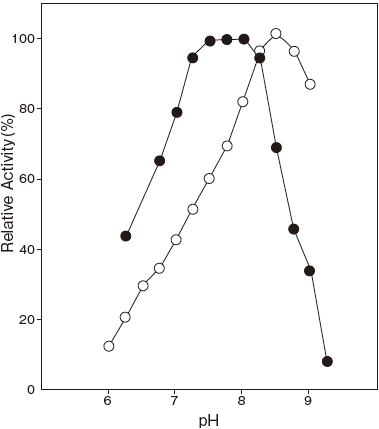
- Optimum pH
- : Serum Acyl–CoA 7.5
: Palmitoyl–CoA 8.5Figure 1
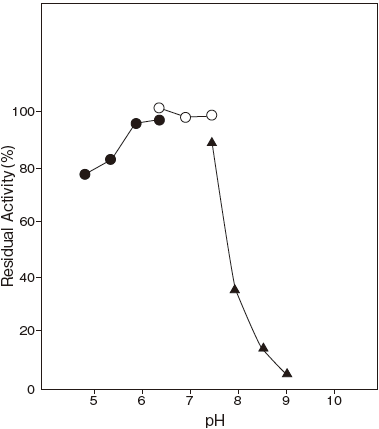
- pH stability
- : 6.0–7.5 (37℃, 60 min)Figure 2
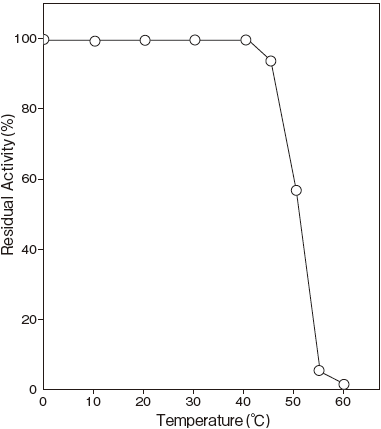
- Thermal stability
- : Stable at 40℃ and below (pH 7.0, 10 min) Figure 3
- Storage stability
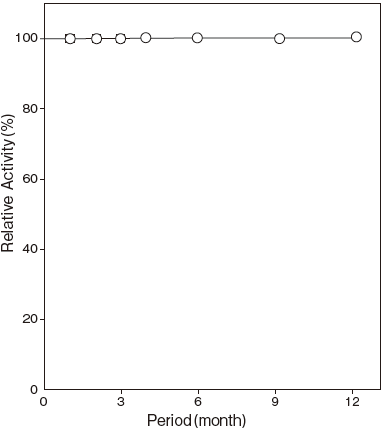
Effects of various - : At least one year at −20℃Figure 4
- chemicals
- : See Table 2
- Stabilizer
- : FAD
- Activator
- : Triton X–100
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of fatty acid when coupled with Acyl–CoA synthetase (T–16).
| ACS | ||
| FFA + CoA + ATP | → | Acyl - CoA + PPi + AMP |
| ACOD | ||
| Acyl - CoA + O2 | → | Enoyl - CoA + H2O2 |
| POD | ||
| 2 H2O2 + 4-AA + Phenol | → | Quinoneimine dye + 4 H2O |
FFA:Free fatty acid
Table 1. Substrate specificity of ACOD
| Substrate | Relative
activity (%) |
Km value (10- 5M) |
|---|---|---|
| Hexanoyl-CoA (6:0) | 11 | |
| Octanoyl-CoA (8:0) | 60 | |
| Decanoyl-CoA (10:0) | 73 | |
| Dodecanoyl-CoA (12:0) | 87 | |
| Tetradecanoyl-CoA (14:0) | 99 | |
| Hexadecanoyl-CoA (16:0) | 53 | 2.0 |
| Octadecanoyl-CoA (18:0) | 16 | 3.8 |
| Icosanoyl-CoA (20:0) | 1 | |
| 9-Tetradecenoyl-CoA (14:1) | 100 | |
| 9-Hexadecenoyl-CoA (16:1) | 65 | |
| 9,12-Hexadecadienoyl- CoA (16:2) | 55 | |
| cis -9-Octadecenoyl-CoA (18:1) | 45 | 4.0 |
| trans -9-Octadecenoyl- CoA (18:1) | 31 | |
| d-12-Hydroxy-trans -9- octadecenoyl-CoA (18:1) | 7 | |
| cis -9, cis -12- Octadecenoyl-CoA (18:2) | 31 | 3.8 |
| cis -6, cis -9, cis -12- Octadecenoyl-CoA (18:3) | 95 | 1.67 |
| 15-Tetracosenoyl-CoA (24:1) | 7 |
Table 2. Effect of various chemicals on ACOD activity
| Additive | Consentration | Relative activity (%) |
|---|---|---|
| None | - | 100 |
| NaCl | 1mM | 102 |
| KCl | 1mM | 104 |
| LiCl | 1mM | 101 |
| NH4Cl | 1mM | 101 |
| MgCl2 | 1mM | 138 |
| BaCl2 | 1mM | 147 |
| CaCl2 | 1mM | 137 |
| MnCl2 | 1mM | 155 |
| ZnCl2 | 1mM | 99 |
| CoCl2 | 1mM | 121 |
| FeCl3 | 1mM | 101 |
| EDTA | 1mM | 74 |
| Triton X-100 | 0.1% | 140 |
| Adekatol SO-120 | 0.1% | 147 |
| Sodium laurylbenzene sulfonate | 0.1% | 54 |
| Sodium laurylsulfate | 0.1% | 43 |
| Deoxycholate | 0.1% | 106 |
Assay
Principle
-
The assay is based on the increase in absorbance at 500 nm as the formation of quinoneimine dye proceeds in the following reactions:
| ACOD | ||
| Palmitoyl–CoA+O2 | → | 2–Hexadecenoyl–CoA+2HO2 |
| POD | ||
| 2H2O2+4–AA+Phenol | → | Quinoneimine dye+4H2O |
Unit definition
-
One unit is defined as the amount of enzyme which generates 1 μmole of H2O2 per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture
0.2 M Tris–HCl buffer pH 8.0 0.20 ml 15 mM 4–AA solution 0.10 ml 0.2% (W/V) Phenol solution 0.10 ml 50 U/ml POD solution1) 0.10 ml
-
1% (W/V) Triton X–100 solution 0.10 ml 5 mM Palmitoyl–CoA solution 2) 0.10 ml Distilled water 0.30 ml 1) : 50 U/ml POD solution
Dissolve 500 U (PPU) of POD with 10 ml of distilled water.2) : 5 mM Palmitoyl–CoA solution
Dissolve 50.3 mg (purity calculation) of palmitoyl– CoA with 10 ml of 10 mM KH2PO4–NaOH buffer pH 7.0. - Enzyme dilution buffer
10 mM KH2PO4–NaOH buffer (pH 7.0) containing 3 mM ATP and 10 μM FAD - Reagents
4–AA: NACALAI TESQUE, INC. Special grade #01907–52
POD: Sigma Chemical Co. Type Ⅱ # P–8250
Triton X–100: The Dow Chemical Company
Palmitoyl–CoA: Asahi Kasei Pharma Corporation
ATP (2Na ・ 3H2O) : Kyowa Hakko Co., Ltd.
FAD (2Na) : Kyowa Hakko Co., Ltd.
ATP: Adenosine triphosphate
FAD: Flavine adenine dinucleotide
Enzyme solution
-
Accurately weigh about 20 mg of the sample and add enzyme dilution buffer to make a total of 20ml. Dilute it with enzyme dilution buffer to adjust the concentration to within 0.2–0.5 U/ml.
Procedure
- Pipette accurately 1.0 ml of reaction mixture into a reaction cuvette (1 ml volume black cuvette) and preincubate at 37℃.
- After 5 min, add 20 μl of enzyme solution and mix to start the reaction at 37℃.
※ In the case of a test blank, add 20 μl of enzyme dilution buffer in place of enzyme solution. - After starting the reaction, measure the rate of increase in absorbance at 500 nm. The rate must be measured within the linear portion of the absorbance curve.
△A/min = (As/min−Ab/min) ≦ 0.060 Abs/minAbsorbance sample : As/min blank : Ab/min
Calculation
Activity (U/mg of powder) = {(△ A/min)/(12.0 × 1/2)} × 1.02/0.02 × 1/x| 12.0 : | millimolar extinction coefficient of quinoneimine dye at 500 nm (cm2 / μmole) |
| 1/2 : | a multiplier derived from the fact that 2 mol of H2O2 produce 1 mol of quinoneimine dye |
| 1.02 : | final volume (ml) |
| 0.02 : | volume of enzyme solution (ml) |
| X : | concentration of the sample in enzyme solution (mg/ml) |
Storage
Storage at −20℃ in the presence of a desiccant is recommended. Enzyme activity will be retained for at least one year under this condition (Figure 4) .
References
- Shimizu, S., Yasui, K., Tani, Y. and Yamada, H. (1979) Biochem. Biophys. Res. Commun., 91 (1) , 108–113.
- Kikuchi, T., Ogawa, M., Ando, M. and Nakagiri, Y (. 1979) Proceedings of Japanese Conference on Biochemistry of Lipids, 21, 144–147.
- Hosaka, K., Kikuchi, T, and Mitsuhida, N. (1979) Proceedings of the Symposium on Chemical Physiology, 19, 180.
- Shimizu, S., Inoue, K., Tani, Y. and Yamada, H. (1980) Anal. Biochem., 107, 193–198.
- Hosaka, K., Kikuchi, T., Mitsuhida, N. and Kawaguchi, A. (1981) J. Biochem., 89, 1799–1803.
- Kawaguchi, A., Tsubotani, S., Seyama, Y., Yamakawa, T., Osumi, T., et al. (1980) J. Biochem., 88, 1481–1486.
ACOD 活性測定法 (Japanese)
試薬液
- 反応試薬混合液
0.2M トリス–HCl 緩衝液pH8.0 0.20 ml
15mM 4–AA 溶液 0.10 ml
0.2% (W/V) フェノール溶液 0.10 ml
50U/ml POD 溶液 1) 0.10 ml
1% (W/V) トリトン X–100 溶液 0.10 ml
5mM パルミトイル–CoA 溶液 2) 0.10 ml
精製水 0.30 ml
1) : 50U/ml POD 溶液 POD500 単位 (PPU) を精製水10ml で溶解す る。 2) : 5mM パルミトイル–CoA 溶液 パルミトイル–CoA 50.3mg (純度換算) を 10mM KH2PO4–NaOH 緩衝液pH7.0 10ml で溶 解する。 - 酵素溶解希釈用液
3mM ATP と10 μM FAD を含む10mM KH2PO4– K2HPO4 緩衝液pH7.0
- 試薬
4–AA:ナカライテスク製 特級 #01907–52
POD:シグマ製 Type Ⅱ #P–8250
トリトンX–100:Dow Chemical 製
パルミトイル–CoA:旭化成ファーマ製
ATP (アデノシン三リン酸・2Na ・3H2O) : 協和発酵製
FAD (フラビンアデニンジヌクレオチド・2Na) : 協和発酵製
酵素試料液
- 検品約20mg を精密に量り、酵素溶解希釈用液に溶 解して全容20ml とする。 その液を酵素溶解希釈用液で0.2~0.5U/ml 濃度とな るように適宜希釈する。
測定操作法
- 反応セル (1ml 用ブラックセル) に反応試薬混合液 1.0ml を正確に分注して37℃で予備加温する。
- 5 分経過後、酵素試料液20 μl を加えて混和し、37℃ で反応を開始する。 ※盲検は酵素試料液の代わりに酵素溶解希釈用液20 μl を加える。
- 反応開始後、500nm における吸光度を測定して直線
的に反応している1 分間当たりの吸光度変化を求め
る。
求められた吸光度変化を試料液はAs/min、盲検液は
Ab/min とする。
ΔA/min = (As/min−Ab/min) ≦ 0.060 Abs/min
計算
-
活性 (U/mg) = {(△ A/min)/12.0 × 1/2)} × 1.02/0.02 × 1/x
12.0 : キノンイミン色素の500nm におけるミリモル分子 吸光係数 (cm2/ μmole) 1/2 : H2O2 2 モルからキノンイミン色素1 モルが生成す ることによる係数 1.02 : 反応総液量 (ml) 0.02 : 反応に供した酵素試料液量 (ml) X : 酵素試料液中の検品濃度 (mg/ml)