CHOLINE OXIDASE [CODⅢ]
from Microorganism
(Choline: oxygen 1-oxidoreductase, EC 1.1.3.17)
Choline + O2 → Betaine aldehyde + H2O2
Betaine aldehyde + O2 + H2O → Betaine + H2O2
Preparation and Specification
- Appearance
- : Yellowish amorphous powder, lyophilized
- Specific activity
- : More than 2 U/mg solid
- Contaminants
- : Alkaline phosphataseLess than 2%(U/U)
- : Glycerophosphorylcholine phosphodiesteraseLess than 2%(U/U)
Properties
- Molecular weight
- : 60 kDa(SDS-PAGE)
- Isoelectric point
- : 5.0 (estimated from amino acid sequence)
- Michaelis constants
- : Choline 6.9 × 10-4M
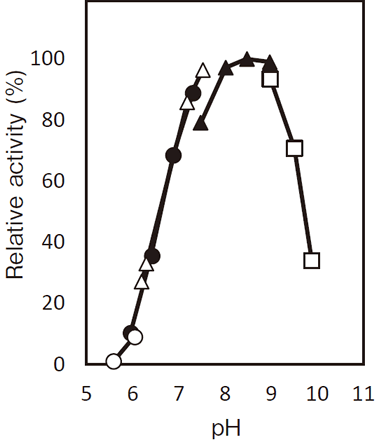
- Optimum pH
- : 7.0–9.0Figure 1
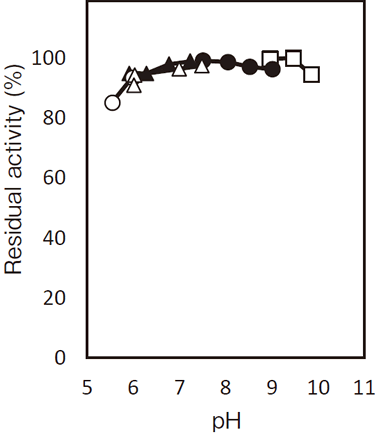
- pH stability
- : 5.5–10.0(37℃, 60 min)Figure 2
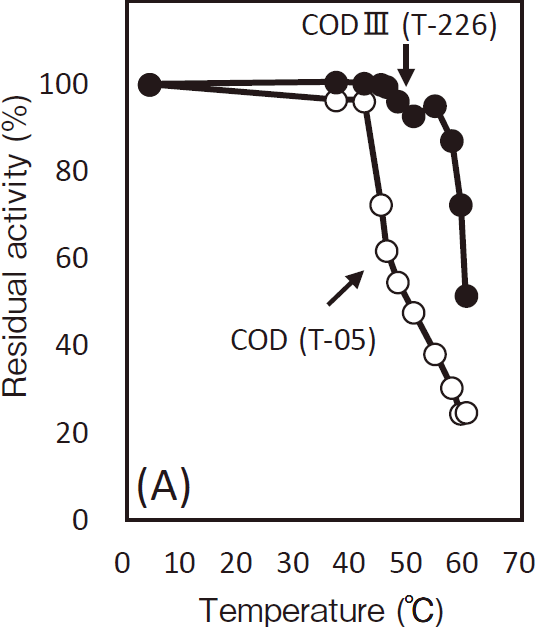
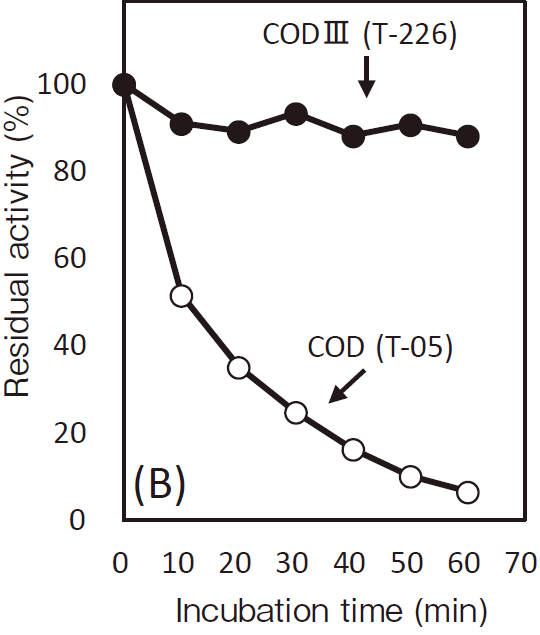
- Thermal stability
- : Stable at 55℃ and below (pH7.0, 30 min)Figure3
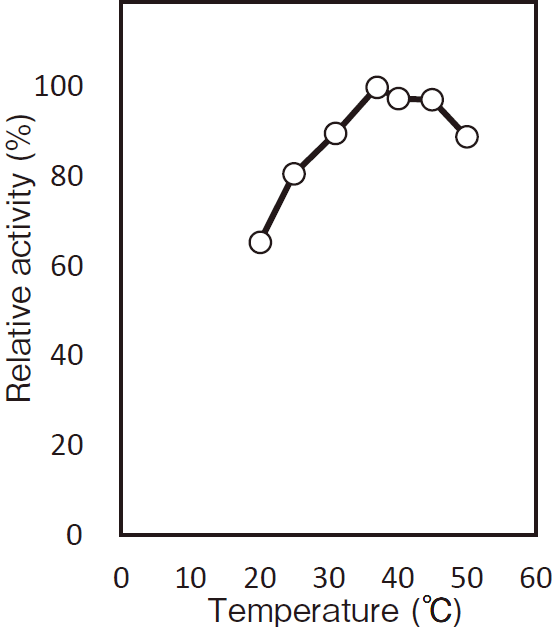
- Optimum temperature
- : 37℃Figure4
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of phospholipids coupled with phospholipase D [PLD Ⅱ (T–222)].
| PLDⅡ | ||
| Phosphatidylcholine + H2O | → | Choline + Phosphatidic acid |
| CODⅢ | ||
| Choline + 2 O2 + H2O | → | Betaine + 2 H2O2 |
| POD | ||
| 2 H2O2 + 4-AA + Phenol | → | Quinoneimine dye + 4 H2O |
Assay
Principle
-
The assay is based on the increase in absorbance at 500 nm as the formation of quinoneimine dye proceeds in the following reactions:
| COD Ⅲ | ||
| Choline+O2 | → | Betaine aldehyde+H2O2 |
| COD Ⅲ | ||
| Betaine aldehyde+O2+H2O2 | → | Betaine+H2O2 |
| POD | ||
| 2H2O2+4-AA+Phenol | → | Quinoneimine dye+4H2O2 |
Unit definition
-
One unit is defined as the amount of enzyme which generates 1 μmole of H2O2 per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture
1.211 g of Tris(hydroxymethyl)amino methane, 2.1 g of choline chloride and 2 ml of 1 %(W/V)phenol are dissolved with 1 N HCl and adjusted to pH 8.0(25℃).
Then, 1 ml of 1 %(W/V)4–AA and 3 ml of 100 PPU/ml POD are added to make a total of 100 ml. - Enzyme dilution buffer
10 mM Tris–HCl buffer(pH 8.0)containing 2 mM EDTA and 1 %(W/V)KCl
EDTA: Ethylenediaminetetraacetic acid - Reagents
Choline chloride: FUJIFILM Wako Pure Chemical Corporation 1st Grade #033–09812
4-AA: NACALAI TESQUE, INC. Special grade #01907–52
POD: Sigma Chemical Co. Type Ⅱ #P–8250
EDTA(2 Na・2H2O): KISHIDA CHEMICAL Co., Ltd. #060–29133
Enzyme solution
-
Accurately weigh about 20 mg of the sample and add enzyme dilution buffer to make a total of 20 ml. Dilute it with enzyme dilution buffer to adjust the concentration as required.
Procedure
- Pipette accurately 3.0 ml of reaction mixture into a small test tube and preincubate it at 37℃.
- After 5 min, add 50 μl of enzyme solution and mix to start the reaction at 37℃.
- After starting the reaction, measure the rate of increase per minutes in absorbance at 500 nm from 2 min to 7 min.
△ A/min ≦ 0.040 Abs/min
Calculation
Activity(U/mg of powder)= {(△ A/min)/(12.0 × 1/2)} × 3.05/0.05 × 1/x| 12.0 : | millimolar extinction coefficient of quinoneimine dye at 500 nm(cm2/μmole) |
| 1/2 : | multiplier derived from the fact that 2 mole of H2O2 produce 1 mole of quinoneimine dye. |
| 3.05 : | final volume (ml) |
| 0.05 : | volume of enzyme solution (ml) |
| X : | concentration of the sample in enzyme solution
(mg/ml) |
Storage
Storage at –20°C in the presence of a desiccant is recommended. Enzyme activity will be retained for at least one year under this condition.
References
- Ikuta, S., Matsuura, K., Imamura, S., Misaki, H. and Horiuchi, Y.(1977)J. Biochem., 82, 157–163.
- Ikuta, S., Imamura, S., Misaki, H. and Horiuchi, Y.(1977)ibid, 82, 1741–1749.
- Ohta–Fukuyama, M., Miyake, Y., Emi, S. and Yamano, T.(1989)J. Biochem., 88, 197–203.
- Takayama, M. et al.(1977)Clin. Chim. Acta, 79, 93.
- Sugawara, K. and Kihara, A.(1978)Eisei Kensa, 27(1), 106–111.
- Okabe, H. et al.(1977)Clin. Chim. Acta, 80, 87.
COD Ⅲ活性測定法(Japanese)
試薬液
- 反応試薬混合液
トリス(ヒドロキシメチル)アミノメタン 1.211gと塩化コリン 2.1g 及び 1%(W/V)フェノール液 2ml を精製水に溶解した後、1N HCl で pH8.0(25℃)に調整し、さらに 1%(W/V)4–AA 溶液1ml と 100PPU/ml POD 溶液 3ml を加えて溶かし、全容 100ml とする。 - 酵素溶解希釈用液
2mM EDTA と 1%(W/V)KCl を含む 10mM トリス− HCl 緩衝液 pH8.0 溶液 - 試薬
塩化コリン:富士フイルム和光純薬製 一級 #033–09812
4-AA:ナカライテスク製 特級 #01907–52
POD:シグマ製 Type Ⅱ #P–8250
EDTA(エチレンジアミン四酢酸・2Na・2H2O): キシダ化学製 #060–29133
酵素試料液
- 検品約 20mg を精密に量り、酵素溶解希釈用液で溶解して全容 20ml とする。その液を酵素溶解希釈用液で適宜希釈する。
測定操作法
- 小試験管に反応試薬混合液3.0ml を正確に分注し37℃で予備加温する。
- 5 分経過後、酵素試料液50 μl を正確に加えて混和し、37℃で反応を開始する。
- 反応開始後、500nm における2 分目から7 分目までの吸光度を測定し 1 分間当たりの吸光度変化を求める。
ΔA/min ≦ 0.040 Abs/min
計算
-
活性(U/mg)= {(ΔA/min)/12.0 × 1/2)} × 3.05/0.05 × 1/x
12.0 : キノンイミン色素の500nm におけるミリモル分子吸光係数(cm2/μmole) 1/2 : H2O2 2 モルからキノンイミン色素1 モルが生成することによる係数 3.05 : 反応総液量(ml) 0.05 : 反応に供した酵素試料液量(ml) X : 酵素試料液の検品濃度(mg/ml)