
Close
FAQ
Close
• High serum Lp-PLA2 activity is a risk factor for coronary artery disease.
• Guidelines*1 recommend the Lp-PLA2 test to predict the risk of future onset of cardiovascular diseases.
• Particularly in the United States and China, the market for Lp-PLA2 tests is growing.
| *1 | 2014 – International Atherosclerosis Society 2016 – American Heart Association/American Stroke Association 2017 – American Association of Clinical Endocrinologists and American College of Endocrinology |
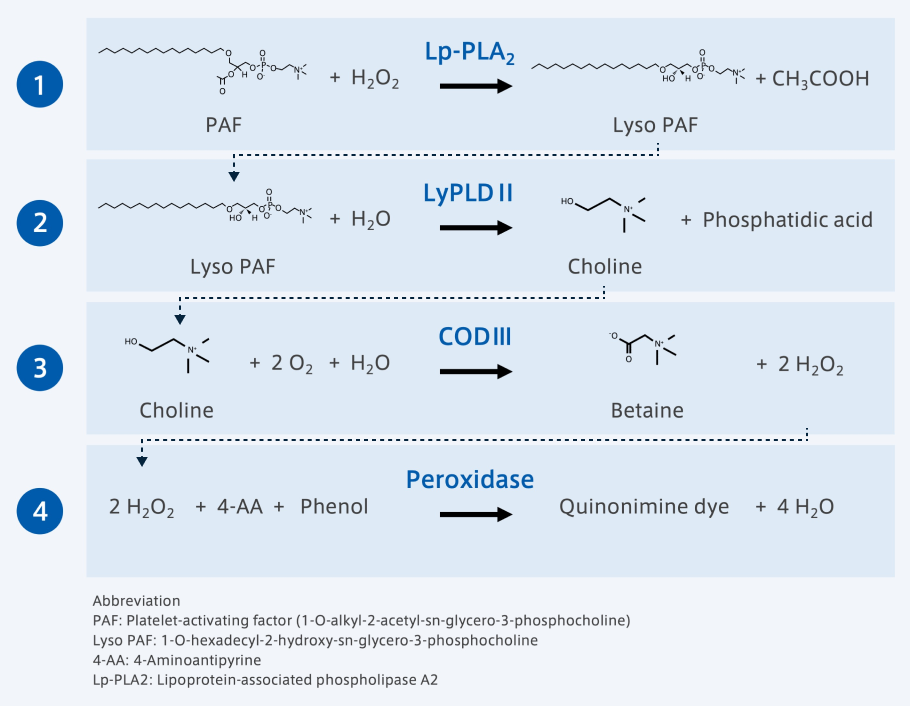
The Lp-PLA2 Activity Assay Reagent (Enzymatic Assay)*2, *3 by Asahi Kasei is characterized by its versatility, which makes it highly convenient, and its endogenous substrate.
*2 Novel enzymatic method for assaying Lp-PLA2 in serum. Clinica Chimica Acta; International Journal of Clinical Chemistry vol. 481 (2018): 184-188. Yamaura, Saki et al. DOI: 10.1016/j.cca.2018.03.012
*3 Patented in Japan, the United States, and China
Versatility |
Asahi Kasei |
Chemical method |
ELISA |
|---|---|---|---|
Number of reagents |
2 |
2–3 |
Many |
Calibration points |
2 |
2–5 |
Many |
Compatibility with general purpose automated biochemical analyzers |
Yes |
Partially |
No |
Reagent stability |
≥12 months |
NA |
NA |
The reagent for the enzymatic assay by Asahi Kasei is stable for 12 months or longer under refrigeration.
We simplify reagent development by supplying the components, materials, and production procedures.
Please contact us if you are looking for other products not listed below.
Asahi Kasei Pharma Diagnostics Division carries many solutions and products.
We may be able to meet your needs.