July 10, 2007 |
Asahi Kasei Pharma Corp.
Asahi Kasei Medical Co., Ltd.
Kuraray Co., Ltd.
Kuraray Medical Inc. |
Revision of plan for integration of medical device businesses
|
| |
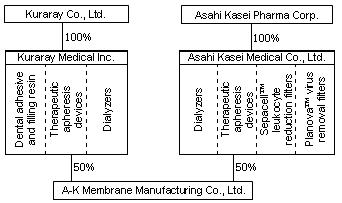
Asahi Kasei Medical (AM), wholly owned subsidiary
of Asahi Kasei Pharma, and Kuraray Medical (KM), wholly owned
subsidiary of Kuraray, have revised their plan for the integration
of medical device businesses as announced on December 14,
2006.
|
| Businesses
subject to integration on October 1, 2007 |
| |
As previously announced:
Dialyzers and therapeutic apheresis devices of AM and
KM. |
| |
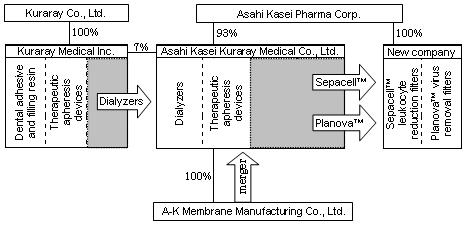
As revised: Dialyzers and
therapeutic apheresis devices of AM and dialyzers of
KM. |
|
| |
| Proportion
of ownership of the integrated company |
| |
As previously announced:
85% by Asahi Kasei Pharma, 15% by Kuraray Medical |
| |
As revised: 93% by Asahi
Kasei Pharma, 7% by Kuraray Medical |
|
| |
| Reason for
revision |
| In the course of prior consultation
with the Japan Fair Trade Commission (JFTC), it became apparent
that a response from the JFTC to the effect that there would
be no concern of restraint on competition in the domestic
market for therapeutic apheresis devices would not be forthcoming
soon enough to enable the planned business integration to
proceed on schedule. AM and KM therefore opted to proceed
first with an integration only of their dialyzer businesses.
The revision of the proportion of ownership of the integrated
company reflects valuation based on the revised scope of businesses
subject to integration. |
| |
| Outlook |
| AM produces devices based on
polysulfone hollow-fiber membrane technology, the global standard
for high-performance clearance of low-molecular-weight proteins,
and devices based on cellulose hollow-fiber technology,
while KM produces devices based on Eval® ethylene-vinyl alcohol
copolymer (EVOH) hollow-fiber technology, featuring outstanding
biocompatibility and enabling mild and gentle therapy for older
patients and those just beginning treatment.
The integration of the two companies' hollow-fiber technology and
know-how will accelerate the development of next-generation devices
which offer greater safety and efficacy, enabling the integrated
operation to enjoy a solid position as a global leader in medical
devices, well placed for expansion and growth. |
| |
| Integration process |
| The Sepacell™ leukocyte reduction filter
and Planova™ virus removal filter businesses
of AM will be transferred to a newly established company.
AM, retaining its dialyzer and therapeutic apheresis device businesses,
will be renamed Asahi Kasei Kuraray Medical Co., Ltd.
The dialyzer businesses of KM will be transferred to Asahi Kasei
Kuraray Medical. KM will retain its dental adhesive and filling resin
business and its therapeutic apheresis device business.
A-K Membrane Manufacturing Co., Ltd., joint venture of AM and KM for
the production of Eval® hollow-fiber membrane for medical devices,
will become a wholly owned subsidiary of Asahi Kasei Kuraray Medical,
and then merged with that company. |
| |
Schematic representation of the business integration
(percentages indicate ownership) |
| |
| Prior to integration |
 |
| |
| Integration |
 |
| |
| Profile of the integrated business |
| Company name: Asahi Kasei Kuraray Medical Co., Ltd. |
| President: To be named from Asahi Kasei Medical |
| Scheduled start of operation: October 1, 2007 |
| Paid-in capital: ¥800 million (tentative) |
| Shareholding: 93% by Asahi Kasei Pharma, 7% by Kuraray Medical |
| Head office: Tokyo, Japan. |
| Business line: |
| |
Development, production,
and sale of dialyzers and therapeutic apheresis products |
| Production sites: Nobeoka, Miyazaki, Japan; Oita, Japan; Hangzhou, Zhejiang, China |
| Sales offices: Japan, US, EU, China, Korea |
| Employees: Approx. 1,400 |
| FY 2007 sales forecast: ¥40 billion (annualized) |
| FY 2010 sales forecast: ¥48 billion |
|
| |
| Asahi Kasei Medical corporate profile |
| President: Yasuyuki Yoshida |
| Establishment: July 1974 |
| Paid-in capital: ¥800 million |
| Shareholding: 100% by Asahi Kasei Pharma |
| Head office: Tokyo, Japan |
| Main products: |
| |
Dialyzers, therapeutic
apheresis products, leukoreduction filters for transfusion,
virus removal filters |
| Production facilities: |
| |
Nobeoka - production of
hollow-fiber dialyzer membrane and other products |
| |
Oita - assembly of dialyzers
and blood filters |
| |
China - assembly of dialyzers |
| Employees: Approx. 1,700 (as of Mar. 31, 2007) |
| Sales: ¥43.1 billion (year ended March 2007) |
|
| |
| Kuraray Medical corporate profile |
| President: Hideo Horii |
| Establishment: June 2001 |
| Paid-in capital: ¥2.5 billion |
| Shareholding: 100% by Kuraray Co., Ltd. |
| Head office: Tokyo, Japan |
| Main products: |
| |
Dialyzers, therapeutic apheresis products, dental adhesive and filling resin |
| Production facilities: |
| |
Kurashiki - production of hollow-fiber dialyzer membrane, dental adhesive, and dental filling resin |
| Employees: Approx. 300 (as of Mar. 31, 2007) |
| Sales: ¥11.8 billion (year ended March 2007) |
|
| |
Eval® is a registered trademark of Kuraray Co., Ltd.
Sepacell™ and Planova™ are trademarks of Asahi Kasei Medical Co., Ltd. |
|