April 9, 2014
Asahi Kasei Corp.
Asahi Kasei ZOLL Medical Corp.
Asahi Kasei ZOLL Medical (AZM), the Asahi Kasei Group company for critical care devices in Japan, will launch its service offering the LifeVest® Wearable Cardioverter Defibrillator throughout Japan on April 15, 2014.
Intended use
Sudden cardiac death (SCD) is the cause of death for approximately 60,000 people per year in Japan. In some cases, it is possible to identify a risk of SCD before it occurs. Being worn continuously (except during bathing), the LifeVest® Wearable Cardioverter Defibrillator provides protection for patients at risk of SCD by constantly monitoring the patient’s heart and, if a life-threatening heart rhythm (ventricular fibrillation or ventricular tachycardia) is detected, delivering a treatment shock to restore normal heart rhythm. The LifeVest® is used to protect a wide range of patients, including patients with newly diagnosed heart failure, following recent myocardial infarction, or following coronary revascularization.
Features
The LifeVest® has one set of electrodes to measure electrocardiogram (ECG) data, and another set of electrodes to deliver the treatment shock. As the detection of life-threatening heart rhythm and delivery of the defibrillation shock are performed automatically, a patient’s life can be saved without assistance of any other person. Furthermore, as the LifeVest® is wearable, its use can be easily initiated or discontinued in accordance with changes in the patient's condition, enabling use to be limited to the period when a patient is at high risk of SCD.
Service
The LifeVest® is rented from AZM to medical institutions, and each patient rents the LifeVest® from a medical institution based on a physician’s prescription. Patients use the LifeVest® while they are in hospital or at home. AZM provides a full range of support services to medical institutions, including education and training on use of the LifeVest®, as well as maintenance and repair services and patient data management systems.
With the launch of the Wearable Cardioverter Defibrillator, AZM provides a new option for defibrillation treatment in Japan, contributing to life and living for more patients.
Outline of the approval
| Approval number: | 22500BZI00017000 | |
|---|---|---|
| Foreign Manufactured Medical Device’s Manufacturing and Marketing Approval Holder: | ZOLL Lifecor Corporation (US) | |
| Designated Marketing Authorization Holder: | Asahi Kasei ZOLL Medical Corp. |
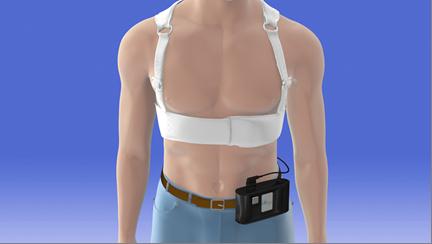
Illustration of LifeVest® being worn
About Asahi Kasei ZOLL Medical (AZM)
A subsidiary of Asahi Kasei Group company ZOLL Medical Corporation, AZM was established in August 2012 to sell ZOLL critical care products in Japan. AZM will continue to seek increased sales in Japan, while working to achieve synergy with the other health care operations of the Asahi Kasei Group.
Adobe Readeris required to view these PDF files.