- WFI
- Separation technology
Introducing next-generation membrane separation technology as an alternative to distillers for the production of Water for Injection (WFI).
Microza responds to various challenges in the WFI application
Massive energy consumption and CO2 emissions from the distiller
Fluctuation in the quality of
distilled water
High initial and
running costs of distiller
Requirement to improve water quality by using other UF membranes
Insufficient filtration performance, short membrane life
Why Microza?
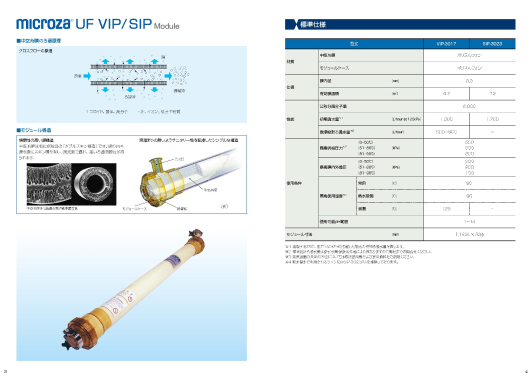
Two different membranes available:
- SIP-3023(capable of hot water disinfection)
- VIP-3017(capable of steam sterilization)
Both have high filtration reliability due to the double-skin structure
VIP-3017 is capable of steam sterilization and water filtration at 80ºC.
Double-pass UF (combination of SIP-3023 and VIP-3017) can be proposed as a complementary process against the risk of membrane rupture.
Microza references in pharmaceutical water industry
Experience and results in the pharmaceutical water industry of
Over 30years
Operating
in the world at
Over 350plants
Microza modules operating in the world:
Over1,700modules
Remote Monitoring-Based Performance Maintenance Service
"Provided as an Equipment System"
We are now offering a new WFI membrane system along with a remote monitoring-based performance maintenance service.
Below is a conceptual picture of our standard WFI system.

Support Details

An Example of Remote Monitoring-Based Performance Maintenance Service
- To monitor operational conditions twice a day via remote monitoring (pressure, flow rate, temperature, tank water level, bioparticles)
- To conduct inspections once every 6 months to maintain stable operation (air leak testing, consumable replacement, steam sterilization)
- To replace membranes every 2 years
Our products

Microza UF SIP-3023, VIP-3017

WFI standard system
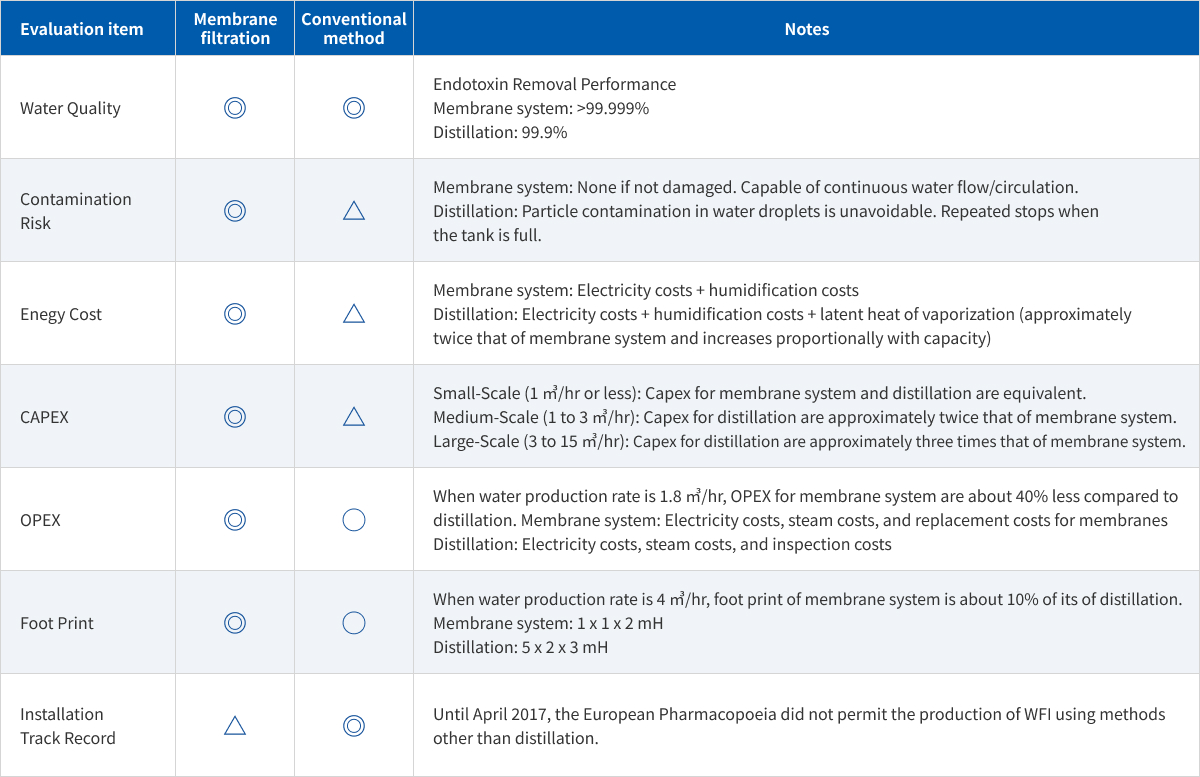
Differences from Conventional Technology
Comparison with Distillers (calculated by Asahi Kasei)
In the manufacturing process of pharmaceutical water, our technology does not just reduce energy consumption but is also expected to lower the risk of contamination and improve cost-effectiveness.
To reduce environmental impact, we propose the transition from distillation to membrane technology as a viable option.