- Features
- Performances
- Specifications
- References
Adsorption Column
| Adsorbent | Material | Styrene divinylbenzene copolymer |
|---|---|---|
| Volume | 350 mL | |
| Priming Volume | 130 mL | |
| Container | Material | Polypropylene |
| Dimension | 220mm[L] x 62mm[D] | |
| Weight | 600 g | |
| Sterilization | High pressure steam | |
Particle Removal Filter
| Filter | Material | Polyethylene (coated with ethylene-vinylalcohol copolymer) |
|---|---|---|
| Area | 0.07 m2 | |
| Container | Material | Poly (vinyl chloride) |
| Dimension | 165mm[L] x 22mm[D] | |
| Priming Volume | 30 mL | |
| Sterilization | Ethylene oxide | |
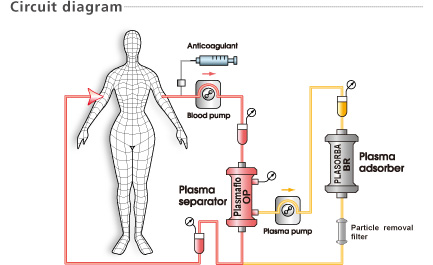
The PLASORBA BR-350 is intended for the treatment of plasma. Never run whole blood through the PLASORBA BR-350. Thrombocytes cannot pass through the PLASORBA BR-350 and may cause blockage. Do not use PLASORBA BR-350 with plasma containing a large amount of thrombocytes.
Trademark
PLASORBA is a trademark of Asahi Kasei Medical Co., Ltd.

PLASORBA BR
Please feel free to send us any questions you may have about our products and support.
Share your feelings and experiences when using our products.