- Features
- Performances
- Specifications
- References
| EC-20W | EC-30W | EC-40W | EC-50W | |
|---|---|---|---|---|
| Hollow Fiber Material | Ethylene vinyl alcohol copolymer | |||
| Inside Diameter | 175 μm | |||
| Wall Thickness | 40 μm | |||
| Surface Area | 2.0 m2 | |||
| Container Material | Polycarbonate | |||
| Dimensions | 280mm[L] x 57mm[D] | |||
| Sterilization | Gamma-Ray | |||
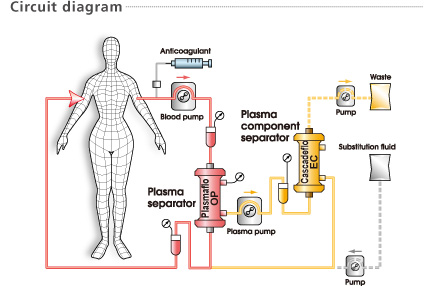
Cascadeflo EC is used as a plasma component separator in double filtration plasmapheresis in combination with a plasma separator (Plasmaflo OP) for selective extracorporeal plasma therapy.
Trademark
Cascadeflo is a trademark of Asahi Kasei Medical Co., Ltd.

Cascadeflo EC
Please feel free to send us any questions you may have about our products and support.
Share your feelings and experiences when using our products.