- Features
- Performances
- Specifications
- References
Cascadeflo XC specifications
| XC-20 | XC-30 | XC-40 | XC-50 | |
|---|---|---|---|---|
| Hollow Fiber Material | Ethylene vinyl alcohol copolymer | |||
| Inside Diameter | 175μm | |||
| Wall Thickness | 40μm | |||
| Surface Area | 2.0m2 | |||
| Priming volume plasma side/filtration side |
110/200mL | |||
| Container Material | Styrene-butadiene block copolymer | |||
| Dimensions | 334mm[L] x 47mm[D] | |||
| Sterilization | Gamma-Ray | |||
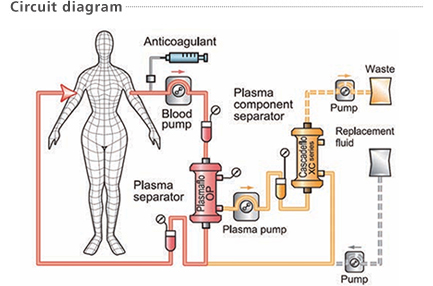
Cascadeflo XC is used as a plasma component separator in double filtration plasmapheresis in combination with a plasma separator (Plasmaflo OP) for selective extracorporeal plasma therapy.
Trademark
Cascadeflo is a trademark of Asahi Kasei Medical Co., Ltd.

Cascadeflo XC
Please feel free to send us any questions you may have about our products and support.
Share your feelings and experiences when using our products.