High-Performance UF Membranes for Pharmaceutical and Medical Device Fields
The Water Treatment Process UF AV/SW Series supports improved purified water quality and process efficiency in the pharmaceutical and medical device fields. The double-skin hollow fiber membrane structure provides high filtration performance and strength. In addition, advanced heat resistance, including steam sterilization compatibility, has led to widespread adoption both domestically and internationally.
Details of Water Treatment Process UF VIP/SIP Series
Features of Water Treatment Process UF VIP/SIP Series
Extensive track record of adoption by pharmaceutical companies and medical device manufacturers both in Japan and overseas.
Large membrane area per unit volume provides high permeate flow and efficient water permeability.
Double-skin structure ensures reliable endotoxin removal.
Compliant with USP Class VI by using components that meet strict medical standards.
The VIP Series is compatible with steam sterilization at 121°C, and the SIP Series is compatible with hot water sterilization at 90°C, providing heat resistance.
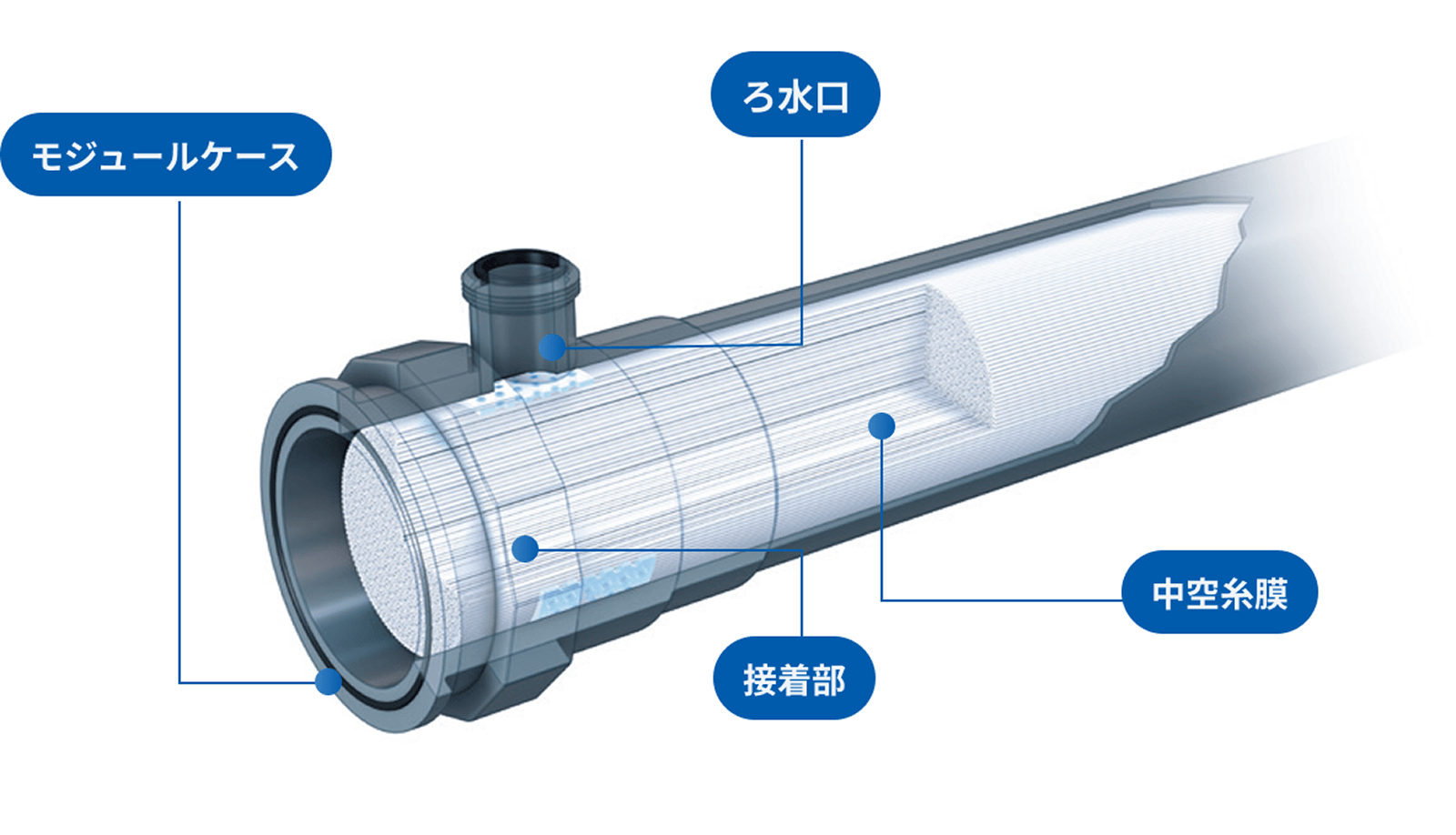
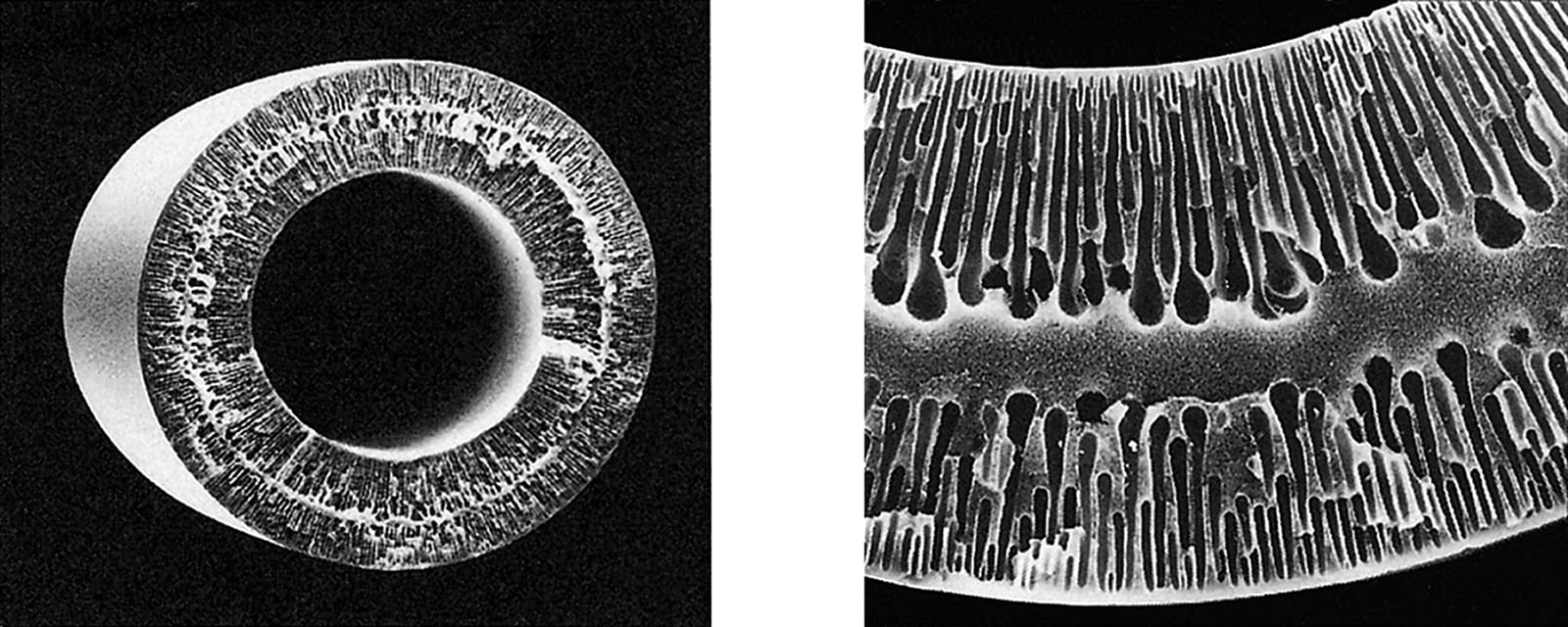
A double skin structure with separation-function skin layers on both inner and outer surfaces ensures high filtration reliability and strength (Learn More Here).
Solutions for Water Treatment Process UF VIP/SIP Series
Application Fields by Industry
Pharmaceutical
Medical Devices
Application Fields by Use
Endotoxin Removal (Learn More Here).
Product Specifications of Water Treatment Process UF VIP/SIP Series
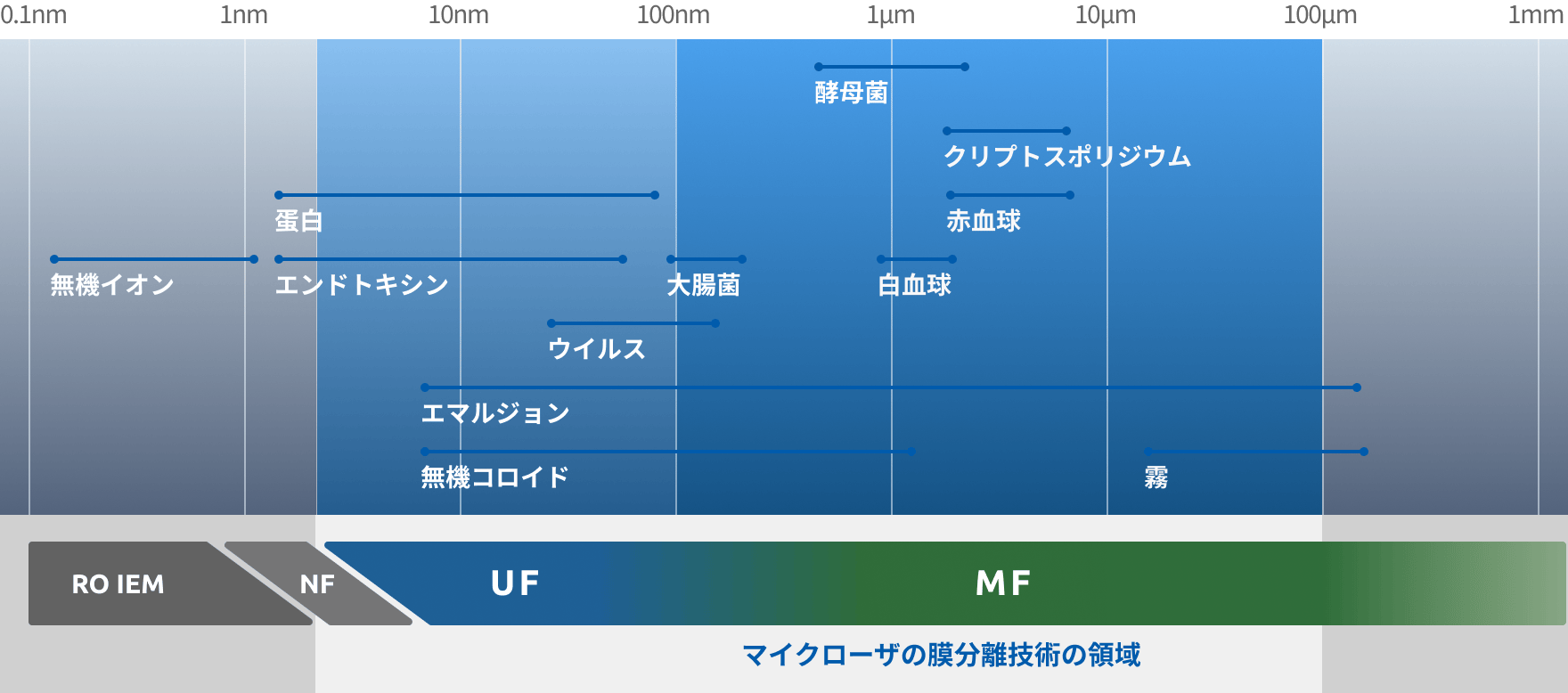
Filtration range of UF membranes capable of removing viruses and macromolecules
Module Structure
Electron micrograph of UF membrane
Datasheet
| Model | VIP-3017 | SIP-3023 | ||||||
|---|---|---|---|---|---|---|---|---|
| Material | Hollow Fiber Membrane | Polysulfone | ||||||
| Module Case | ||||||||
| Specification | Membrane I.D. (mm) | 0.8 | ||||||
| Effective Membrane Area (m2) | 4.7 | 7.2 | ||||||
| Performance | Nominal Molecular Weight Cut-Off | 6,000 | ||||||
| Initial Permeate Flow Rate*1 (L/hour at 100 kPa) | 1,000 | 1,700 | ||||||
| Standard Design Filtration Flow Rate*2 (L/hour) | 500–800 | - | ||||||
| Operating Conditions | Max. Feed Pressure (kPa)*3 | (0–50°C) | 500 | |||||
| (51–80°C) | 300 | |||||||
| (81–95°C) | 200 | |||||||
| Max. Transmembrane Pressure (kPa) | (0–50°C) | 300 | ||||||
| (51–80°C) | 200 | |||||||
| (81–90°C) | 100 | |||||||
| Max. Operating Temperature (°C) | Normal Use | 80 | ||||||
| Hot Water Sterilization | 95 | |||||||
| Sterilization | 125 | - | ||||||
| Operating pH Range | 1–14 | |||||||
| Module Dimensions (mm) | 1,129 L x 89 φ | |||||||
| *1: Indicates the initial permeate flow rate when filtering clarified water at 25°C and 100 kPa. *2: Standard design flow rate may vary depending on raw water quality and design conditions. Please contact us for details. *3: For details on steam sterilization methods, refer to the instruction manual and technical documentation. | ||||||||