From Distillation to Microza®
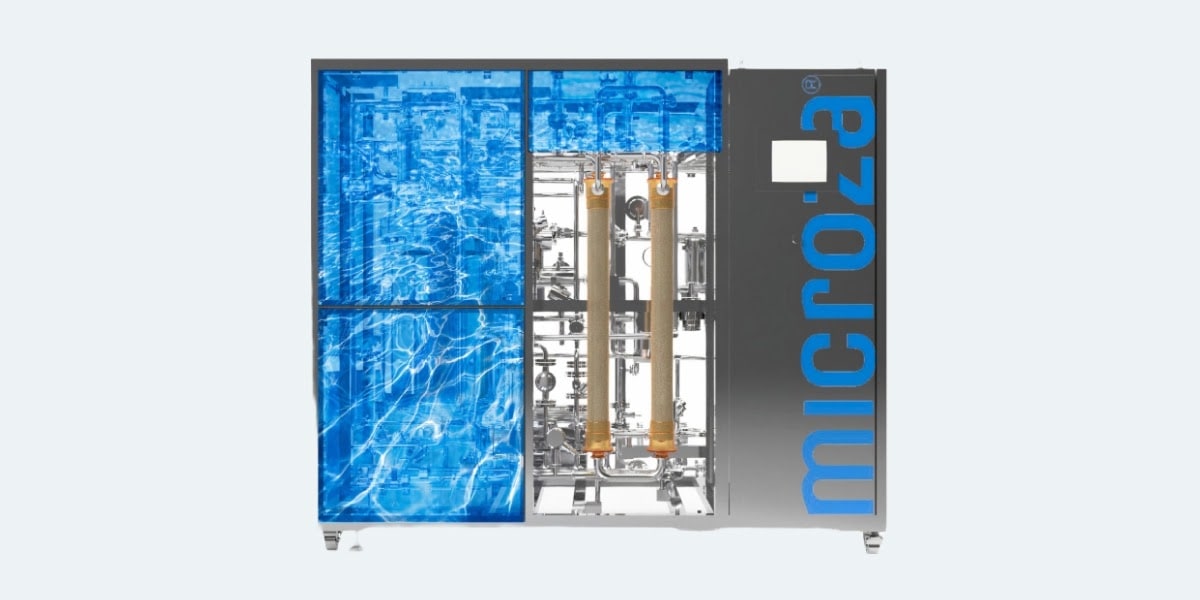
Microza® solutions utilizing UF membrane technology enable endotoxin removal while stabilizing high-quality water supply. This greatly improves efficiency and sustainability in pharmaceutical operations. In addition, the introduction of new system WFI (Water for Injection) equipment enables remote monitoring and membrane replacement optimization, reducing maintenance burden and operational costs.
Stable High Water Quality and Reduced Risk of Foreign Matter Contamination
In pharmaceutical water production, distillers and UF membrane modules are used. These methods can present challenges such as water quality deterioration, foreign matter contamination, and insufficient filtration performance leading to shorter service life. These issues are significant risk factors as they directly affect product and research quality.
To minimize these risks and ensure stable high water quality, Microza® provides UF membranes featuring a double-skin structure (dual-layer membrane). With Microza®, this structure enhances filtration accuracy and reliability. Furthermore, a backup process combining dual-pass UF with SIP-3023 (hot water sterilizable) and VIP-3017 (steam sterilizable) helps reduce the risk of membrane rupture.
Learn More About Microza® Technologies Such as the Double-Skin Structure
Energy Consumption and CO2 Contributes to Emission Reduction
Distillers used in pharmaceutical and research facilities are widely utilized as a means to obtain high-quality water suitable for endotoxin removal. However, distillers consume large amounts of energy and CO2 emissions have become a significant issue.
With Microza® UF filtration for endotoxin removal, not only is high filtration accuracy expected, but energy consumption and CO2 emissions can be significantly reduced compared to conventional distillation methods. This technology addresses the growing demand for decarbonization across society.
Reduced Initial and Operating Costs
The introduction of distillers tends to involve high initial costs, and maintenance after installation also results in significant operating costs. Labor and expenses for tasks such as Class 1 pressure vessel inspections further increase costs. In addition, frequent and labor-intensive maintenance can reduce the efficiency of facility management, which is a challenge for many companies.
Microza® solutions using hollow fiber membrane modules help balance cost efficiency and maintenance. No large-scale equipment is required, and high water quality is achieved while reducing initial capital investment, significantly improving cost-effectiveness.
Microza® Enables High-Temperature Sterilization
In pharmaceutical and biotechnology operations, sterilization using hot water or steam is often required. The Microza® UF model “VIP-3017” can withstand water temperatures up to 80°C and is also compatible with steam sterilization. This ensures flexible temperature and hygiene control while maintaining equipment durability and supporting long-term stable operation.
Comprehensive Services to Maintain Operational Performance
To maintain a high-quality water supply via UF membrane filtration over the long term, it is essential to ensure stable operational performance while minimizing maintenance workload and the risk of issues. Microza® provides services combining remote monitoring, periodic inspections, and membrane replacement to support continued system operation after installation.
-
Regular Monitoring of Operating Status via Remote SupervisionRemote monitoring is conducted twice daily for parameters such as pressure, flow rate, temperature, tank water level, and bio-particles. This supports early detection of abnormalities and ensures stable membrane system operation.
-
Maintain Stable Operation with Semiannual Comprehensive InspectionsRegular implementation of air leak inspections, consumable replacement, and steam sterilization helps prevent issues and keeps equipment in optimal condition.
-
Membrane Replacement Every Two YearsThis supports long-term performance maintenance and reduces unexpected downtime and quality risks.
Launch of New WFI (Water for Injection) Equipment
Based on Asahi Kasei's hollow fiber membrane technology, an advanced water treatment solution for efficient, high-purity production of water for injection (WFI) has been launched.
Learn More About WFI EquipmentExtensive Track Record and Service System for Reliable Use
Microza® has over 30 years of experience in pharmaceutical-grade water and operates more than 350 sites globally. Based on this proven expertise, over 10,000 pharmaceutical water systems have been implemented. As demonstrated by this extensive track record, Microza® provides continuous support from installation through operation to ensure long-term system reliability.