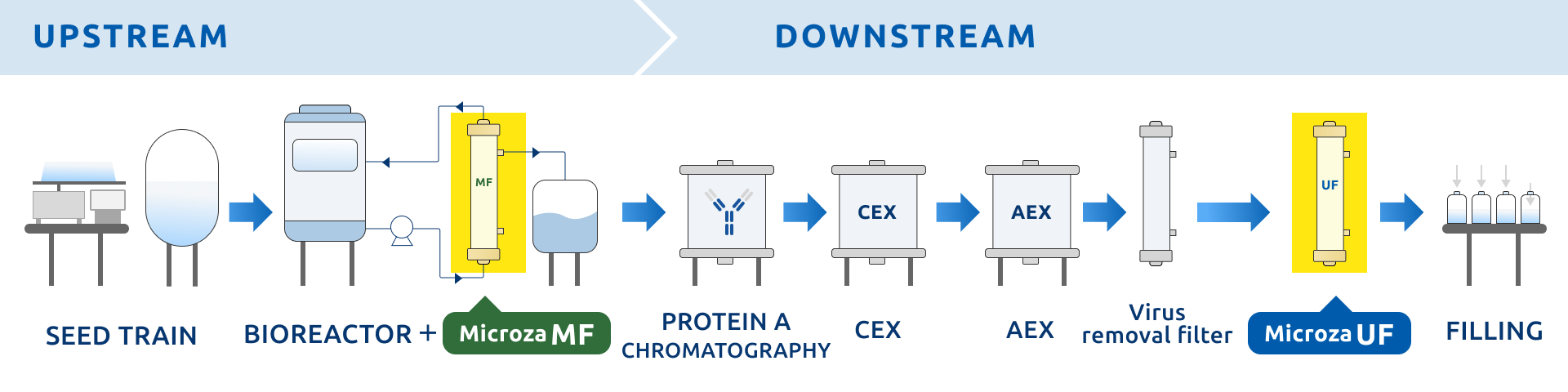
Strongly Supporting High Quality and Efficiency in Pharmaceutical Production
Proprietary technologies meet all requirements for high yield, high efficiency, and high hygiene management in pharmaceutical manufacturing. They support goal achievement from cultivation to purification, providing solutions that combine economic efficiency and stability. From membrane technology to after-sales service, comprehensive support is provided to address customer challenges.
Improving Yield and Separation Efficiency in Production Processes
In biopharmaceutical production, a major challenge is how to improve both yield and separation efficiency from cultivation to purification for various targets such as animal cells, E. coli, yeast, viruses, vaccines, and enzymes.
For example, minimizing loss and shortening processing time are required in each process, including antibody recovery in animal cell perfusion culture, protein separation from E. coli or yeast, virus and vaccine concentration and purification, and enzyme concentration and desalting. Filtration technologies that enhance both yield and efficiency are essential to meet these demands.
To address these challenges, Microza® provides solutions with proprietary membrane formation technologies and a wide variety of membrane materials. It delivers stable filtration performance in both upstream and downstream processes and achieves high yield and separation efficiency of target substances, thereby improving overall productivity. For example, PVDF membranes provide high antibody permeability in perfusion culture processes. PE membranes enable protein separation without adsorption in culture media. PAN membranes offer excellent separation performance for enzyme concentration and purification due to a combination of material properties (high hydrophilicity and low adsorption) and uniform pore structure.
Ensuring Sanitary Performance to Meet Strict Hygiene Standards
Strict hygiene control is essential in pharmaceutical production. Repeated steam sterilization and cleaning processes place significant stress on equipment, making material selection critical to minimize deterioration and contamination risk.
Microza® PVDF membranes withstand steam sterilization while maintaining durability, making them well-suited to meet stringent hygiene requirements. In addition, PE membranes offer high hydrophilicity and excellent durability, making them resistant to fouling and easier to maintain in a clean condition. By leveraging the properties of both steam-sterilizable PVDF membranes and fouling-resistant PE membranes, Microza® delivers sanitary performance that meets the strict standards required in pharmaceutical applications.
Achieving High Operational Stability
In pharmaceutical manufacturing, long-term and continuous stable operation is essential. Microza® offers high filtration stability and maintains performance over long periods, enabling reduced maintenance frequency and lower risk of system shutdown. Additionally, Microza® PE membranes feature high hydrophilicity and excellent cleanability, helping reduce downtime due to cleaning and significantly contributing to sustained productivity and cost savings. This operational stability minimizes production losses from equipment downtime and helps reduce maintenance-related costs.
Active in a Wide Range of Pharmaceutical Manufacturing Applications
Microza® has a proven track record in pharmaceutical production environments. It consistently delivers high performance across various process stages, including purification and concentration of culture and fermentation fluids, separation of antibodies and proteins, and endotoxin removal, earning high praise in the field.
| Application | Category | MF | UF | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Membrane Material | PVDF | PE | PAN | PS | ||||||||||||
| Pore Size / MWCO | 0.2 µm | 0.65 µm | 0.1 µm | 0.25 µm | 6 kDa | 13 kDa | 50 kDa | 80 kDa | 3 kDa | 6 kDa | 10 kDa | |||||
| Product Name | UMP | UJP | PSP | PMP | AIP | ACP | AHP | AOP | SEP | SIP | VIP | SLP | ||||
| Membrane I.D. (mm) | 1.4 | 1.1 | 0.7 | 1.9 | 0.7 | 0.8 | 0.8 | 1.4 | 0.8 | 0.8 | 0.8 | 0.8 | 1.4 | 0.8 | 1.4 | |
| Cell Culture purification or perfusion | ○ | ○ | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Fermentation Broth purification | ○ | ○ | ○ | ○ | ○ | - | - | - | - | - | - | - | - | - | - | |
| Microorganisms, Yeast and Suspended Solids removal | - | - | ○ | ○ | ○ | - | - | - | - | - | - | - | - | - | - | |
| Amino Acids and Antibiotics purification | - | - | ○ | ○ | ○ | - | - | - | - | - | - | - | - | - | - | |
| Enzyme and Protein purification/ concentration | - | - | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | - | ○ | ○ | - | - | |
| Colloidal Substances removal | - | - | - | - | - | ○ | ○ | ○ | ○ | - | - | - | - | - | - | |
| API purification | - | - | - | - | - | - | - | - | - | - | - | ○ | ○ | - | - | |
| Pyrogen and Endotoxin removal | - | - | - | - | - | - | - | - | - | - | ○ | ○ | ○ | ○ | - | |